Research Article
SMOFlipid versus Intralipid in Postoperative ICU Patients
Ayman Anis Metry1*, Wail Abdelaal1, Milad Ragaei2, Mona Refaat1, and George Nakhla2
1Assistant professor of anesthesiology, Ain Shams University, Cairo, Egypt
2Lecturer of anesthesiology, Ain Shams University, Cairo, Egypt
Corresponding author
Ayman Anis Metry, Assistant Professor of anesthesiology, Ain Shams University, Cairo Egypt, E-mail: drayman_metri@med.asu.edu.eg
Received Date: 19th November 2014
Accepted Date: 05th December 2014
Published Date: 08th December 2014
Citation
Metry AA, Abdelaal W, Ragaei M, Refaat M, Nakhla G (2014) SMOFlipid versus Intralipid in Postoperative ICU Patients. Enliven: J Anesthesiol Crit Care Med 1(6): 015.
Copyright
@ 2014 Dr. Ayman Anis Metry. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim of the work
Lipids are important components of total parentral nutrition, especially for patients after major abdominal surgery. Traditionally used intralipid has many complications and can lead to increased infection rate and sepsis, that is why, it is not indicated in cases with low immunity and sepsis. So, in this study, we compared the effect of intralipid and SMOFlipid on the level of IL-6, in addition to lipid profile, liver enzymes, coagulation profile and renal functions.
Patients and Methods
This prospective, randomized, double-blinded study was designed to compare between two groups of postsurgical ICU patients. Group I and group II had 42 and 41 patients respectively. Both the groups were given total parentral nutrition for not less than 7 days postoperatively.
Group I was given Intralipid as a source of fat, and Group II was given SMOFlipid in substitution of intralipid. Vital signs (including blood pressure, heart rate, and body temperature), blood liver function test, renal function test, coagulation profile, white blood cells (WBCs), and lipid profile (triglycerides [TGs], cholesterol [CH], low-density lipoprotein [LDL], and high-density lipoprotein [HDL]) were monitored. The assessments for IL-6 was performed which indicate inflammatory response. The clinical outcomes, including morbidity, mortality, and infectious complications during the hospital stay, were also evaluated.
Results
The study showed no significant differences between the two groups with regard of vital signs and chemical profiles for cholesterol, triglycerides and liver enzymes.
IL 6 levels were significantly different between the two groups on day 4 and 7. IL-6 was significantly lower in SMOFlipid group on day 4 and 7 than in intralipid group.
Conclusion
On comparing intralipid versus SMOFlipid, we have discovered that SMOFlipid group showed low level of IL6 which is as a single agent gives an indication of reduced inflammatory response with SMOFlipid but with a weak proof and need more studies for bigger scale of inflammatory indicators.
Keywords
Total Parentral Nutrition (TPN); SMOFlipid, intralipid; IL-6
Abstract
Aim of the work: Lipids are important components of total parentral nutrition, especially for patients after major abdominal surgery. Traditionally used intralipid has many complications and can lead to increased infection rate and sepsis, that is why, it is not indicated in cases with low immunity and sepsis. So, in this study, we compared the effect of intralipid and SMOFlipid on the level of IL-6, in addition to lipid profile, liver enzymes, coagulation profile and renal functions.
Patients and Methods: This prospective, randomized, double-blinded study was designed to compare between two groups of postsurgical ICU patients. Group I and group II had 42 and 41 patients respectively. Both the groups were given total parentral nutrition for not less than 7 days postoperatively.
Group I was given Intralipid as a source of fat, and Group II was given SMOFlipid in substitution of intralipid. Vital signs (including blood pressure, heart rate, and body temperature), blood liver function test, renal function test, coagulation profile, white blood cells (WBCs), and lipid profile (triglycerides [TGs], cholesterol [CH], low-density lipoprotein [LDL], and high-density lipoprotein [HDL]) were monitored. The assessments for IL-6 was performed which indicate inflammatory response. The clinical outcomes, including morbidity, mortality, and infectious complications during the hospital stay, were also evaluated.
Results: The study showed no significant differences between the two groups with regard of vital signs and chemical profiles for cholesterol, triglycerides and liver enzymes.
IL 6 levels were significantly different between the two groups on day 4 and 7. IL-6 was significantly lower in SMOFlipid group on day 4 and 7 than in intralipid group.
Conclusion On comparing intralipid versus SMOFlipid, we have discovered that SMOFlipid group showed low level of IL6 which is as a single agent gives an indication of reduced inflammatory response with SMOFlipid but with a weak proof and need more studies for bigger scale of inflammatory indicators.
Introduction
Postoperative care of patients underwent major surgeries necessitates infusion of total parentral nutrition in which lipid infusion is one of its constituents. Intravenous lipid emulsion is not only supplying energy through the essential fatty acids contained but also these essential fatty acids affect the immune system and may lead to immunosuppression and excessive inflammation [1].This effect is quit important in critically ill patients [1,2] and it may be the main leading cause of organ failure which is the main cause of death among ICU patients.
Linoleic acid which is ω-6 polyunsaturated fatty acid (PUFA) is known to be immunosuppressive and may lead to an increased risk of infection. Intralipid (soybean oil–based lipid emulsions) is rich in linoleic acid, which is in addition to depressing cell-mediated immunity may lead to promotion of inflammation, primarily via the production of proinflammatory eicosanoids (ie, leukotrienes, prostaglandins, and thromboxanes) by arachidonic acid (AA). [1] Fish oil-based emulsions contain mainly long-chain ω-3 polyunsaturated fatty acids, consisting of 18-carbon α-linolenic acid, 20-carbon eicosapentaenoic acid (EPE) and 22-carbon docosahexaenoic acid (DHA) plus a small amount of α-linolenic acid. The emulsions in question are not neutral for the immune system. EPA and DHA easily penetrate the cell interior and are components of tissues. They modify lipid membranes; affect the profile of synthesized eicosanoids by their increased production with EPA instead of arachidonic acid (AA). EPA-based eicosanoids and inflammation mediators are less active [3,4]. Emulsions based on fish oil have also inhibitory effects on signal transduction and expression of genes involved in the inflammation. In patients with sepsis, the use of fish oil-based emulsions resulted in reduced concentrations of pro-inflammatory cytokines IL-6 and IL-10. Fish oil was found to modify significantly the cytokine profile and to increase the EPA levels in serum [5]. Moreover, its use was demonstrated to enhance the production of DHA and EPA metabolites without affecting the production of AA, whose products show pro-inflammatory effects [4]. The use of fish oil shortened the hospitalization of patients after major abdominal surgeries compared to patients receiving soybean oil-based emulsions [4].
It has been argued that the ratio of ω −6 to ω−3 PUFAs in parentral lipids, to support the immune system, should mirror the nutritional environment in which human evolution took place [6,7]. This view is bolstered by observations in an animal transplant model in which the infusion of an emulsion with a ratio of ω −6 to ω −3 PUFAs of ≈2:1 showed immune-neutral characteristics, in the form of a maximally reduced graft organ survival, whereas graft survival gradually increased with both lower or higher ratios of ω −6 to ω −3 PUFAs [7,8]. In line with these findings, a novel emulsion has been developed. This so-called SMOFlipid (Fresenius-Kabi) is a 20% lipid emulsion with the lipid being a mix of 30% MCT, 30% SO, 25% OO, and 15% FO, resulting in a ratio of ω −6 to ω −3 PUFAs of 2.5:1.
In this study, we studied the effect of traditional Intralipid versus SMOFlipid on interleukin-6, which is considered the main cytokines increasing with inflammatory process, in postoperative patients needing total parentral nutrition.
Patients and Methods
This prospective, randomized, double-blinded study was approved by the Ethics Committee of Ain Shams University hospitals. Ninety consecutive patients admitted to the SICU after major operations were enrolled into this study. Patients were recruited between September 2012 and April 2014. Informed written consent was obtained from all patients. They were randomized to receive PN with the same volume and calories of glucose, nitrogen, and fat but different lipid components, either Intralipid (group I) or SMOFlipid (group II).
Exclusion criteria were allergy to egg, soybean protein, or other content of the lipid emulsion; general contraindication to parentral therapy: acute lung edema, overhydration, or cardiopulmonary insufficiency; pregnancy or breastfeeding; severe coagulopathy; [5] shock; diabetes mellitus with ketoacidosis presented within 7 days; Acute Physiology and Chronic Health Evaluation II (APACHE II) score >25; abnormal renal function (serum creatinine>1.4 mg/dL); abnormal liver function (alanine aminotransferase [ALT] >60 IU/L or total bilirubin >1.2 mg/dL); type IV hyperlipidemia, disorder of lipid metabolism, or hypertriglyceridemia (>354 mg/dL); unconsciousness or uncooperativeness; or participation in any other clinical study within 1 month.
Patients were assigned to the intervention or control group by the institutional intensivists by use of a computer-generated block randomization list. Both the patients and the investigators were thus unaware of the infused drug. Group I was defined as the control group and group II the experimental group. Postoperatively, all patients received PN for more than consecutive 7 days through an indwelling central venous catheter or peripheral catheter. Glucose, amino acids (Aminosteril 10% for intravenous infusion; Fresenius Kabi Deutschland GmbH, Bad Homburg vor der Höhe, Germany), fat- and water-soluble vitamins, and trace elements were provided to both groups by infusion pumps for 12-16 hours daily. Total calories were calculated for each patients in both groups and 30-40 % of this calories were given as lipid with one condition, that, fat content does not exceed 1.5 g fat/kg body weight (BW) /day. The lipid emulsions were given separately with the infusion pump to control the infusion duration for 12-16 hours (not exceeding 0.125 g fat/kg (BW)/h) from 8:00 am to 8:00 pm or 12am. In group I, Intralipid (20% Fresenius Kabi Deutschland GmbH, Bad Homburg vor der Höhe, Germany) was given. In group II, the lipid content of PN was partially replaced by fish oil (SMOFlipid 20% emulsion for intravenous infusion; Fresenius Kabi Deutschland GmbH). The nutrition in both groups was isonitrogenous and isocaloric. (SMOFlipid 20% contains Fish Oil 30 g; Medium Chain Triglycerides 60 g; Olive Oil 50 g; Soya Oil 60g / l. Intralipid 20% contains Egg Phospholipids 1.2 g; Glycerol 2.2 g; Soya Oil 20 g / 100 ml).
Safety and efficacy were evaluated comprehensively. Vital signs (including blood pressure, heart rate, and body temperature), blood liver function test, renal function test, coagulation profile, white blood cells (WBCs), and lipid profile (triglycerides [TGs], cholesterol [CH], low-density lipoprotein [LDL], and high-density lipoprotein [HDL]) were monitored. The assessments for IL-6 was performed which indicate inflammatory response. The clinical outcomes, including morbidity, mortality, and infectious complications during the hospital stay, were also evaluated. For laboratory measurements, 12 mL of whole blood (8 mL serum, 4 mL EDTA) was withdrawn before PN was started as the baseline data and on the fourth and seventh days, respectively, after PN use (termed postoperative day [POD] 0, POD4, POD7). Routine blood test and biochemistry analysis were immediately performed at the clinical laboratory of Ain Shams University Hospital according to standard procedures. Serum vials for analysis of IL-6 were separated and kept at 2–8°C and measured in 24 hours. For quantitative detection of cytokine, OX40 ligand, and G-CSF, enzyme immunoassays were performed according to the manufacturer’s instructions with an enzyme-linked immunosorbent assay (ELISA) kit commercially available from R&D Systems (Minneapolis, MN).
Data are presented as mean ± SD, unless indicated otherwise. Statistical analyses were performed using SPSS version 14 (SPSS, Chicago, IL, USA). One factor ANOVA was used to analyse changes over time within a treatment group. Student’s t-test was used for comparisons between time points and for comparisons between groups at a particular time point; equal variances were not assumed. Linear correlations were determined as Pearson’s correlation coefficients. In all cases, a value of P < 0.05 was taken to indicate statistical significance.
Results
In group I, 3 patients did not continue the study because they stopped PN before continued 7 days and in Group II, 4 patients did not continued the study either because of stoppage of PN or complications. So group I had 42 patients and group II 41 patients.
There was no significant difference between the two study groups regarding age, sex, body mass index, simplified acute physiology score, organ failure score or diagnosis upon admission to surgical ICU (table 1).
| GI (Intralipid) | GII (SMOF) | ||
| Age (years) | 58.2±11.3 | 56.8±10.8 | |
| Sex (male/female)* | 22/20 (52.33/47.67) | 23/18 (56.1/43.9) | |
| BMI (kg/m2)@ | 28.1±2.1 | 27.9±1.9 | |
| SAPS II# | 46.7±5.2 | 43.5±4.8 | |
| Organ failure score | 8.7±1.1 | 8.9±1.3 | |
| Diagnosis* | CVS | 6(14.28) | 4(9.7) |
| Respiratory | 4(9.5) | 8(19.5) | |
| Renal | 2(4.7) | 4(9.7) | |
| CNS | 2(4.7) | 2(4.8) | |
| Metabolic | 8(19.04) | 6(14.63) | |
| GIT | 8(19.04) | 6(14.63) | |
Table 1 characteristics of study population
@BMI body mass index
# SAPS Simplified Acute Physiology Score
Data presented as mean ±SD
* Data presented as number (percent)
P value > 0.05 nonsignificant
The contents in both regimens of daily parentral nutrition were exactly similar except slightly higher medium chain triglycerides to long chain triglycerides (1.5 vs. 1.2)and lower fish oil (0 vs. 0.3) in intralipid regimen over SMOF regimen (table 2).
| Total Calories, | Glucose, | Amino | MCT/ | Fat | FO | Ca, mEq/ | Mg, mEq/ | Zn, mEq/kg | Cl, mEq/kg | Na, mEq/ | K, mEq/kg | |
| kcal/kg BW | g/kg BW | Acid, g/kg | LCT (SO) | g/kg BW | kg BW | kg BW | BW | BW | kg BW | BW | ||
| BW | Mixture | |||||||||||
| GI (Intralipid) | 35 | 6 | 1.2 | 1.5 | 1.5 | 0 | 0.12 | 0.12 | 1.92 × 10−3 | 2.4 | 1.2 | 0.72 |
| GII (SMOF) | 35 | 6 | 1.2 | 1.2 | 1.5 | 0.3 | 0.12 | 0.12 | 1.92 × 10−3 | 2.4 | 1.2 | 0.72 |
Table 2 Contents in the Regimen of Daily Parentral Nutrition.
BW, body weight; FO, fish oil; LCT, long-chain triglyceride; MCT, medium-chain triglyceride; SO, soybean oil. 1 gm fat gives 9 k calories and 1 gm glucose gives 3.4 k calories.
The vital signs named blood pressure both systolic and diastolic, pulse, respiratory rate and temperature showed a non statistically significant difference between the two study groups at admission, day 4 and day 7 after admission to surgical ICU (table 3).
| GI (Intralipid) | GII (SMOF) | P | ||
| SBP | Baseline | 163± 87 | 151 ± 88 | >0.05 NS |
| D4 | 158± 98 | 150±87 | ||
| D7 | 159±88 | 156±79 | ||
| DBP | Baseline | 102±37 | 110±54 | >0.05 NS |
| D4 | 100±41 | 102±55 | ||
| D7 | 102±42 | 106±54 | ||
| Pulse | Baseline | 114±32 | 123±42 | >0.05 NS |
| D4 | 115± 33 | 122±44 | ||
| D7 | 117±38 | 124±45 | ||
| RR | Baseline | 21±12 | 25±13 | >0.05 NS |
| D4 | 21±11 | 24±13 | ||
| D7 | 20±12 | 24±13 | ||
| temp | Baseline | 39.3±3.1 | 39.9±4.1 | >0.05 NS |
| D4 | 38.5±3.2 | 37.9±3.8 | ||
| D7 | 37.2±3.2 | 37.5±3.6 | ||
| TG | Baseline | 96.7 ± 7.3 | 89.1 ± 8.5 | >0.05 NS |
| D4 | 121.6±12.8 | 116.7±12.7 | ||
| D7 | 122.4±13.4 | 116.5±12.6 | ||
| CH | Baseline | 199.6±11.9 | 194.3±12.4 | >0.05 NS |
| D4 | 214.5±16.7 | 207.4±13.7 | ||
| D7 | 213.9±16.6 | 204.6±14.8 | ||
| LDL | Baseline | 102.4±10.3 | 110.3±9.6 | >0.05 NS |
| D4 | 112.5±14.5 | 108.6±9.7 | ||
| D7 | 112.4±14.3 | 110.1±10.6 | ||
| HDL | Baseline | 59.6 ± 2.9 | 61.1±2.7 | >0.05 NS |
| D4 | 59.5 ± 3.9 | 59.8±3.2 | ||
| D7 | 58.6 ± 2.8 | 60.5±2.8 | ||
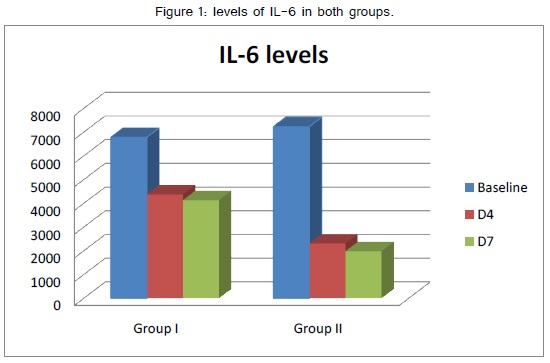
| IL6 | Baseline | 6786±4581 | 7234±5323 | >0.05 NS |
| D4 | 4379±3245 | 2314±1123 | <0.05 S | |
| D7 | 4137±3435 | 1986±1021 | <0.01 HS |
Table 3 Vital signs, lipid profile and IL6 measurment among study groups
The lipid profile named triglycerides, cholesterol, low density lipoprotein and high density lipoprotein showed a non-statistically significant difference between the two study groups at admission, day 4 and day 7 after admission to surgical ICU (table 3).
IL6 showed a non-statistically significant difference between both study groups at admission but showed a significantly lower level at day 4 of admission and highly significant lower level at day 7 of admission in SMOF group than intralipid group (table 3, figure 1).
The laboratory parameters named TLC, AST, ALT, bilirubin, creatinine and PTT showed a non statistically significant difference between the two study groups at admission, day 4 and day 7 after admission to surgical ICU (table 4).
| GI (Intralipid) | GII (SMOF) | P | ||
| TLC | Baseline | 15.3±7.2 | 17.2±7.8 | >0.05 NS |
| D4 | 13.6±6.7 | 14.1±7.1 | ||
| D7 | 11.5±6.6 | 12.3±7.0 | ||
| AST | Baseline | 88±52 | 76±51 | >0.05 NS |
| D4 | 65±43 | 61±34 | ||
| D7 | 41±23 | 51±32 | ||
| ALT | Baseline | 69±42 | 55±41 | >0.05 NS |
| D4 | 45±33 | 47±31 | ||
| D7 | 39±21 | 41±29 | ||
| Bilirubin | Baseline | 1.9 ± 0.6 | 2.1 ± 0.8 | >0.05 NS |
| D4 | 2.1 ± 0.7 | 2.0 ± 0.7 | ||
| D7 | 2.1 ± 0.8 | 2.1 ± 0.7 | ||
| Creatinine | Baseline | 1.8 ±0.9 | 2.1 ±0.9 | >0.05 NS |
| D4 | 2.1 ±1.1 | 2.1 ±1.0 | ||
| D7 | 2.2 ±1.1 | 2.1 ±1.1 | ||
| PTT | Baseline | 47.9 ±15.9 | 39.9 ±13.8 | >0.05 NS |
| D4 | 56.2 ±16.8 | 48.6±17.3 | ||
| D7 | 62.2 ±17.8 | 58.7±17.9 |
Table 4: Routine laboratory parameters in the two treatment groups
The clinical outcomes including duration of ventilation, days of ICU stay, Days of hospital stay, 1 week mortality and 1 month mortality showed a non-statistically significant difference between the two study groups (table 5).
| GI (Intralipid) | GII (SMOF) | P | |
| Ventilated days | 7.2±4.3 | 6.5±5.1 | >0.05 NS |
| ICU days | 10.4±6.2 | 11.7±7.2 | >0.05 NS |
| Hospital stay | 15.7±11.4 | 19.4±12.6 | >0.05 NS |
| 1 week mortality | 2(4.7) | 3(7.3) | >0.05 NS |
| 1month mortality | 3(7.1) | 3(7.3) | >0.05 NS |
Table 5 Clinical outcomes in the two treatment groups
Discussion
A recent phase I study reported that a short infusion (6 h) of SMOF at a rate of 0.125 g fat/kg body weight per hour in healthy male volunteers, when compared with pure SO (Lipovenoes; Fresenius-Kabi), was well tolerated and increased plasma elimination, as evidenced by a less marked increase in serum triacylglycerol concentration and, at the end of infusion, lower serum triacylglycerol concentrations [9]. This is the same dose of lipid infusion used in our study with the same result of triacylglycerol concentration.
We also found that, the lipid profile in addition to triglycerides as cholesterol, low density lipoprotein and high density lipoprotein showed a non-statistically significant difference between the two study groups at admission, day 4 and day 7 after admission to surgical ICU.
Surgical trauma could induce a general inflammatory response associated with a stimulation of the innate immune system and a depression of cell-mediated immunity. Moreover, perioperative lipid supplement for those who have undergone a major operation with temporary gut dysfunction may aggravate this disarrangement [10]. Fatty acids can modulate the immune and inflammatory response in vitro and in vivo studies [11]. Patients with indication for parentral nutrition receive fatty acids (FA) as lipid emulsions (LE) for parentral administration. Depending on the fatty acids composition, LEs can have different impacts on immune functions, and thus affect the patient’s clinical course [12]. A meta-analysis using data from both surgical and critically ill patients suggested that the use of conventional lipid emulsions with the major component of ω-6 PUFA is associated with higher complication rates [13].
The hyper-inflammatory state may be regulated by substrate availability. The immunomodulation of ω-3 fatty acids in contrast to the ω-6 fatty acids is recognized for the ability to modify leukocyte activity, alter lipid-mediator generation, and modulate cytokine release [14].
Intravenous infusion of fish oil rapidly leads to an incorporation of ω-3 fatty acids in leukocyte cell membrane phospholipids, leading to a reduced production of proinflammatory cytokines because of a higher ratio of ω-3 to ω-6 fatty acids [15].
Leukotrienes have numerous effects on inflammatory and immune functions, such as leukocyte-endothelial interaction, lymphocyte proliferation, and induction of cytokine gene expression (eg, IL-1, IL-6, or TNF-α). Recently, novel ω-3 fatty acid–derived products of neutrophil-endothelial interaction, which are exclusively formed by dioxygenation from EPA or DHA, have been identified in murine models as well as in human plasma. Named resolvins and neuroprotectins, these mediators are potently anti-inflammatory and inflammation resolving and are shown to play an important role in improving mortality in a murine model of colitis [16].
Mayer et al. [17] displayed a significant improvement in neutrophil function in patients receiving ω-3 fatty acids, including Leukotrienes generation and respiratory burst. Liang et al also showed decreased IL-6, elevated CD4+/ CD8+ ratio, and higher CD3+ and CD4+ lymphocyte percentage in colorectal cancer patients receiving postoperative ω-3 fatty acid-supplemented PN. These findings suggest that supplementation of ω-3 fatty acids may support immunocompetent cells under inflammatory conditions such as surgical trauma [17].
Jacintho et al. [18] in 2009 compared the effect of fish oil-based (FO) lipid emulsions (LE) for parentral administration with standard LE and a new FO containing LE composed of four different oils on the antigen presentation and inflammatory variables. They found that All LE decreased the HLA-DR and increased CD28 and CD152 expression on monocytes/macrophages and lymphocytes surface (p < 0.05). SO/FO and MCT/ SO/FO decreased lymphocyte proliferation (p < 0.05). All LE decreased IL-2 production, but this effect was enhanced with MCT/SO/FO and SMOF (p < 0.05). MCT/ SO/FO decreased IL-6 and increased IL-10, whereas SO had the opposite effect (p < 0.05). They concluded that fish oil based lipid emulsion (FO LE) inhibited lymphocyte proliferation and had an anti-inflammatory effect. These effects seem to be enhanced when FO is mixed with MCT/SO. SMOF had a neutral impact on lymphocyte proliferation and IL-6 and IL-10 production [18]
In our study the IL6 showed a significantly lower level at day 4 of admission and highly significant lower level at day 7 of admission in SMOF group than in intralipid group, and this result goes with the results of the previously shown studies.
Heller et al. [19] demonstrated that no coagulation and platelet abnormalities were evoked by fish oil supplementation as high as 0.2 g/kg/d for 5 postoperative days [19]. Improved gas exchange as well as inflammatory cytokine modification was displayed by Barbosa et al. [5], by including fish oil for septic ICU patients. Improved liver and pancreas function parameters were also observed in postoperative cancer patients [20]. Interestingly, fish oil-derived emulsions have been reported to prevent PN-associated liver disease (PNALD) [21] and treat essential fatty acid deficiency [22].
Effects of SMOF and SO on liver function and oxidative stress have been compared in metabolically stressed patients, with SMOF showing slightly dampened liver enzyme abnormalities and increased plasma concentrations of antioxidants [23]. A double-blind, randomized study compared TPN based on SMOF or on SO in patients for 5 d after major abdominal surgery [24]. In our study the laboratory parameters named TLC, AST, ALT, bilirubin, creatinine and PTT showed a non-statistically significant difference between the two study groups at admission, day 4 and day 7 after admission to surgical ICU.
In this study, we found that the clinical outcomes including duration of ventilation, days of ICU stay, days of hospital stay, 1 week mortality and 1 month mortality showed a non- statistically significant difference between the two study groups, although it was less in SMOFlipidgroup.
The lower magnitude of postoperative inflammatory response to the use of ω-3 fatty acids may have a favorable impact on clinical outcomes of patients after major surgery. A large prospective, multicenter trial conducted by Wichmann et al. [25] randomized the surgical patients requiring intensive care to receive 5 days of PN, including soybean oil or a mixed soybean LCT/ MCT/fish oil emulsion formula. The latter group had significant increases in EPA, LTB5 production, and antioxidants, as well as significantly shorter lengths of hospital stay. A lower tendency of a postoperative infection rate was also observed in the SICU patients with the ω-3 fatty acid supplement in this study [25].
Our results, as regards days of hospital stay, goes with the previous study and also with Schulzki et al. [26] who discovered that, SMOF, administered at a dose of 1.5 g fat/kg body weight per day, was well tolerated and increased plasma ω-3 FA concentrations and decreased ω-6 FA concentrations. Neutrophil leukotriene B5 release was enhanced on day 6 with SMOF, and the length of hospital stay decreased by 7 d (13 compared with 20 d). These data corroborate findings in an earlier study, in which the length of hospital stay in post-gastrointestinal surgery patients decreased more with SMOF than with SO (13 compared with 20 d) [26]. A recent trial randomly assigned 200 patients after elective abdominal or thoracic surgery to receive TPN based on either SMOF or SO for 5 d postoperatively [27]. Although both emulsions were well tolerated and relevant laboratory variables were not different between groups, a trend toward a reduced length of hospital stay was observed with SMOF (16 compared with 18 d). These differences in days of hospital stay, between our study and these studies, may be related to the difference in the dose of infusion of SMOFlipid, as we used lower dose and soothe difference in the level of neutrophil leukotriene B5 release.
Also our results does not go with Tsekos et al., who showed a significantly decreased mortality rate, as well as a significantly shorter hospital stay, in patients receiving pre- and postoperative fish oil supplements compared with standard PN [28].
Alonso and colleagues in 2013 studied twelve representative ICU’s participated in a nutrition survey. The survey was divided in two sections: A) Management of artificial nutritional support in critically ill patients and B) Assessment of a new parentral nutrition formulation adapted to critically ill patients. They found that 50% tried to reduce volume of PN and 100% of them had an insulin infusion protocol. 39% of prescribers recommended high-protein, low-volume and low-glucose TPN; 42% prescribe TPN with SMOF (soybean, MCT, olive and fish oil); and 33% with OOBE (olive oil based emulsion) as lipid emulsion. 92% added glutamine. 60% considered that the new formulation may be indicated for sepsis, trauma, burn patients and MOF (multiple organ failure) and the 30% would use it as a routine therapy at the time of admission. 40% considered that insulin requirements were reduced; 50% claimed better volume management and 60% highlighted the protein/volume ratio. Attending to patient outcome, patients receiving the specific formulation have less affected hepatic function, higher protein intake and lower volume infusion but no significant differences were observed and they required less insulin dosage (p = 0.07) [29].
In our study, the vital signs named blood pressure both systolic and diastolic, pulse, respiratory rate and temperature showed a non-statistically significant difference between the two study groups at admission, day 4 and day 7 after admission to surgical ICU.
Conclusion
On comparing intralipid versus SMOFlipid, we have discovered that SMOFlipid group showed low levels of IL6 than in intralipid group which is statistically significant and this goes with many other previous researches. As IL-6 measurements per se cannot give a sharp suggestion of reduced inflammation and only a weak clue, so we suggest repeating the study with measurements of other cytokines as IL-1, IL-8, IL-10 in addition to TNF-α, this in addition to comparing different doses of SMOFlipid to detect the optimum dose of SMOFlipid with optimum reduction of inflammatory process.