Case Report
Renaud Poncin1, Aurore Lafosse2, Pauline Richer3, Christine Galant4, Thierry Duprez5, Liliane Marot6, Frank Cornelis7, Benoit Lengelé8, and Jean-Francois Baurain9*
1Medical Oncology Department, Cliniques Saint-Pierre, Ottignies, Belgium
2Plastic Surgery Department, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Bruxelles, Belgium
3Dermatology Department, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Bruxelles, Belgium
4Anatomopathology Department, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Bruxelles, Belgium
5Radiology Department, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Bruxelles, Belgium
6Dermatology Department, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Bruxelles, Belgium
7King Albert II Cancer Institute, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Bruxelles, Belgium
8Plastic Surgery Department, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Bruxelles, Belgium
9King Albert II Cancer Institute, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Bruxelles, Belgium
Corresponding author
Jean-Francois Baurain, King Albert II Cancer Institute, Cliniques universitaires Saint-Luc, Université catholique de Louvain, Bruxelles, Belgium, E-mail: jf.baurain@uclouvain.be
Received Date: 09th March 2015
Accepted Date: 14th May 2015
Published Date: 18th May 2015
Citation
Poncin R, Lafosse A, Richer P, Galant C, Duprez T, et al. (2015) Treatment of Dermatofibrosarcoma Protuberans with Imatinib before Minimal Surgery could Prevent Local Relapses. Enliven: Clin Dermatol 1(1): 006.
Copyright
@ 2015 Dr. Jean-Francois Baurain. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Dermatofibrosarcoma protuberans (DFSP) is cutaneous soft tissue sarcoma affecting mainly young adults and characterized by an indolent slow dermal growth leading to late diagnosis when large tumours cause important aesthetics and functional impairments. Local relapses due to insufficient excision are common. Therefore, complete excision of the primary tumour with free margins is the cornerstone therapeutic option with subsequent potential mutilations decreasing the quality of life patients.
The genetic landmark of DFSP is a unique chromosomal translocation displacing the promoter of the collagen type1 α1 next to the platelet-derived growth factor (PDGF) gene. This leads to an unregulated production of PDGF activating the tumour growth after binding on its dedicated receptors. Based on the pathogenesis of this lesion, the option of treating DFSP patients with imatinib mesylate, a tyrosine kinase inhibiting this loop, has raised.
We have treated consecutively four patients affected by a dermatofibrosarcoma with imabinib mesylate in order to downsize the tumour before surgery. All of them responded and curative surgery was thereafter performed. No relapse was observed after a median follow up of 32 months. Therefore, our results assess the value of using Imatinib as neoadjuvant treatment of DFSP and we put into perspectives with the existing literature observations.
Keywords
Dermatofibrosarcoma protuberans; DFSP; Imatinib; PDGFR; Neoadjuvant
Introduction
Dermatofibrosarcoma protuberans (DFSP) is a rare skin sarcoma accounting for approximately 1% of sarcomas and 0.1% of all cancers [1]. These tumours are often diagnosed in patients in 3rd or 4th decade. The most common locations of dermatofibrosarcoma are on the trunk, the lower and upper extremities, and the head and neck, respectively 47%, 20%, 18% and 14% of cases [2]. DSFP is characterized by an indolent slow dermal growth. This tumour has irregular shapes and finger-like extensions thereby easily invading underlying structures such as fascia, muscle or bone [3]. The diagnosis is frequently late at a time when tumours have a wide extension. The standard treatment of DFSP is local excision. In old series where minimal resections were performed, up to 60% of the patients recurred with a local relapse occurring up to 2-3 years after the surgery. If a wide excision was performed with macroscopic negative margins of at least 3 cm, local recurrences fall to 10%. The resection of the primary tumour may lead to aesthetic and functional impairment because of its size and local aggressiveness, particularly in young patients due to the poor laxity of skin. Moreover, the head and neck area can be very difficult to treat in view to preserve the required margin while protecting essential anatomic units. Metastases are seldom (5%) in the standard form of DFSP and when present are mainly localised in lung.
DSFP has a unique pathological feature with an infiltration of dermis and subcutaneous tissue with storiform spindle cells interdigitating with fat and adnexal tissue forming a honeycom-like structure [4]. The nuclear mitotic rate is low to moderate [5]. These spindle cells are typically CD34+ on immunohistochemistry and constitute a clue in the diagnosis [6]. At present, we don't know if the spindle cells have a fibroblastic origin or if they derived from circulating hematopoietic progenitor cells [7,8]. DFSP can transform into a more aggressive fibrosarcomatous variant (FS-DFSP) which is associated with a higher frequency of metastasis in the range of 10-15%, a higher rate of local recurrence and also with a more rapid dermal growth [9,10].
DFSP is also associated with a unique genetic rearrangement between chromosome 17 and 22 resulting in unbalanced translocation t(17q22;22q13) usually in the form of a supernumerary ring chromosome. This rearrangement leads to fusion of the promoter of collagen type I aI chain (COL1A1) gene to the platelet-derived growth factor b chain (PDGFB) gene. This genetic abnormality is the landmark of DFSP but also of FS-DFSP, and the related infantile form of giant cell fibroblastoma. Therefore, the identification of this translocation by FISH or RT-PCR can be helpful in the differential diagnosis of poorly differentiated skin sarcoma [11]. This rearrangement leads to an overproduction of PDGFB. The PDGF-BB homodimer has the highest affinity for PDGF receptor b (PDGFRB). Upon its activation, the kinase domain of PDGFRB will promote cell activation. Since PDGFRB is present on the cell surface of DFSP, an autocrine or paracrine loop will induce endless cell proliferation. Tyrosine kinase inhibitors could block the activation of PDGFRB.
The imatinib mesylate (Glivec®, Novartis) is a small molecule that inhibits intracellular tyrosine kinase domain of different proteins such as BCR-ABL or c-Kit. The drug was first used in chronic myeloid leukemia (CML) and in gastro-intestinal stromal tumors (GIST). In 2001, it has been observed that in vitro imatinib mesylate could inhibit the tyrosine kinase activity of PDGFRB and was successfully used for the treatment of DFSP [12,13]. Since 2006, imatinib mesylate was approved for the treatment of metastatic or unresectable dermatofibrosarcoma. Subsequently, imatinib mesylate was used to treat patients before surgery (neoadjuvant treatment) for large primary tumours, especially in the head and neck localization, with the aim to reduce the size of the tumour to allow a less invasive surgical resection resulting in limited postoperative disfigurement and functional impairment. During the period from November 2005 to October 2011 we have treated 26 DFSP at the Centre du Cancer of the Cliniques universitaires Saint-Luc. Among those, 4 patients with a large tumour were elected and received imatinib mesylate as neoadjuvant treatment. Novartis gave imatinib mesylate in a compationnal program accepted by local ethics committee. All patients underwent a complete tumour resection after at least 4 months of treatment. We hereby report our series and make a review of the literature.
Case Report
We have treated four patients with imatinib mesylate in the neoadjuvant setting. All these patients were considered to be inoperable in a first intention due to functional or aesthetic disorder so we thought that it would be a good indication for a neoadjuvant treatment. In all our four patients, we saw all the histopathologic and immunohistochemic features of DFSP like dermis infiltration with spindle cells harbouring CD34+ in the cellular membrane. We didnâ??t search the COL1A1-PDGFB transcript in the biopsy because it was not routinely realised in Belgium at the time of the inclusion. A summary of the clinical features and treatment overview of these patients is provided in Table 1.
|
|
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
|
Age (Years) |
47 |
29 |
34 |
62 |
|
Sex |
F |
M |
M |
F |
|
Tumour Location |
Left preauricular |
Presternal |
Skullâ??s top |
Right lumbar |
|
Size (before treatment) in mm |
26 x 16 x 25 |
22 x 48 x 5.5 |
90 x 50 x 7 |
59 x 40 x 45 |
|
Treatment duration (months) |
10 |
4 |
4 |
4 |
|
Grade 3-4 adverse events |
none |
none |
none |
none |
Table 1: Characteristics of the 4 DFSP patients treated with Imatinib
Abbreviation: DFSP, dermatofibrosarcoma protuberans.
Patient 1
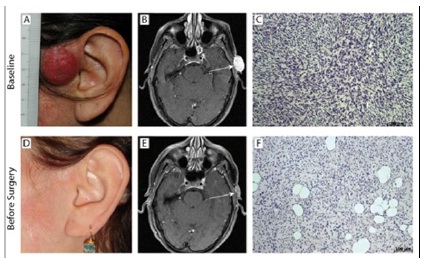
In May 2005, a 47 year-old woman presented a preauricular nodular lesion. This lesion slowly increases and 6 months later a biopsy was finally performed (Figure 1). The pathologist concludes to a dermatofibrosarcoma. Due to the localization and the tumour size (26x16x25mm), we decided to start a neoadjuvant treatment with imatinib mesylate at a dose of 400mg/d in December 2005. After 4 months of treatment, the MRI showed a partial response. Treatment was continued but no further regression was observed on the preoperative MRI (12x1.5x13mm). Therefore after 10 months of a well-tolerated treatment, the remaining lesion was removed with macroscopic free margins of 15mm. The pathologic report shows free margins of at least 3mm in the lateral extremities without subcutaneous fascia infiltration in depth. Seventy-one months after the surgery, the patient is still in complete remission.
Figure 1: Tumour response in patient #1 treated with Imatinib
A 47 year-old woman treated for 10 months with Imatinib. The clinical, radiological and histological evolutions of her DFSP is shown comparing pre-Imatinib baseline status (upper row) and post-Imatinib status (lower row) before curative surgery. The clinical pictures of her tumour before (panel A) compared to after 10 months of Imatinib (panel D) show a clinical complete remission. The MRI at baseline shows a subcutaneous tumour mass on T1-Gadolinium (panel B) and a small residue after treatment with significantly decreased Gadolinium enhancement (panel E). The histological analysis of baseline tumour shows all the characteristics of DFSP (panel C) and the analysis of the resected tumour (panel F) shows fibrosis and lesser cellularity.
Patient 2
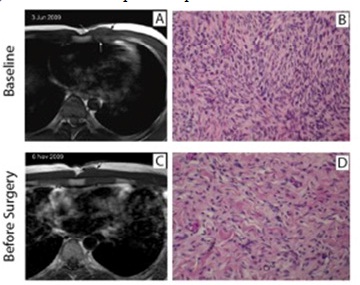
A 29 year-old patient was seen at the plastic surgery consultation in March 2009 with a cutaneous nodular lesion of 48mm localised in the presternal area. An excisional biopsy of this lesion was performed and showed a histologic pattern compatible with a dermatofibrosarcoma protuberans. Within few weeks, we saw around the scar multiple new lesions corresponding to in-transit metastases or to an early multifocal relapse of the dermatofibrosarcoma. New biopsies were performed confirming the relapse/progression. An MRI confirms the in-depth infiltration of the pectoral muscle. In front of this clinical situation, we decided to start a neoadjuvant treatment with imatinib mesylate at a dose of 400mg/d. After two months of treatment, the disappearance of one in-transit metastasis and the regression of all others were observed. An MRI of the thoracic wall confirmed a slight decrease (40mm vs 48mm) of the biggest lesion and a scar appearance of the other lesions (Figure 2). The treatment was well tolerated and we decided to continue the same treatment for two more months. No further decrease was observed. Therefore, a complete resection of the lesions was performed with minimal margins. The pathological report confirms the diagnosis of DFSP and all the margins were free but the pectoral margin was only 1 mm. The patient is still in complete remission 34 months after the complete resection of his DFSP.
Figure 2: Tumour response in patient #2 treated with Imatinib
A 29 year-old man treated for 4 months with Imatinib. We report here the radiological and histological evolutions of his DFSP comparing pre-Imatinib baseline status (upper row) and post-Imatinib status (lower row) before curative surgery. The MRI at baseline shows a prepectoral tumour mass on pre-contrast T1-weighted MR images in the axial-transverse incidence (panel A) and a moderate but significant shrinkage on similar view after treatment (panel C). The histological analysis of baseline tumour shows all the characteristics of DFSP (panel B) and the analysis of the resected tumour (panel D) shows a decrease in cellularity and an increase of the collagen matrix.
Patient 3
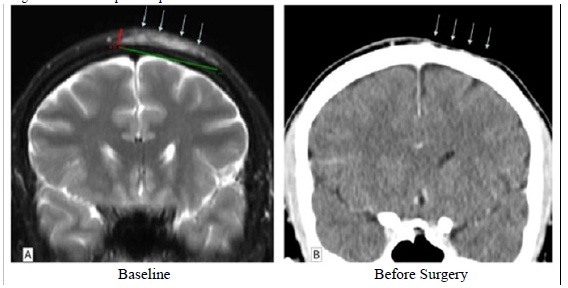
In September 2010, a 34 year-old man presented at the dermatology consultation with a cystic lesion of skullâ??s top developing since 2005. An incisional biopsy was performed confirming the diagnosis of DFSP. On the MRI performed at the time of the biopsy, the tumour was 7mm thick and a 50mm transversal extent. We decided to start in November 2010 a neoadjuvant treatment with imatinib mesylate at a dose of 400mg/d. The first CT-scan evaluation after 4 months of treatment showed a dramatic tumour shrinkage corresponding to a clinical complete response (Figure 3). A surgical excision was then performed with lateral macroscopic margins of 50mm. Paradoxically with the clinical and the radiological impression, the surgical specimen showed a large lesion corresponding to a dermatofibrosarcoma with all free lateral margins. On the pathological analysis, we donâ??t see any subcutaneous or fascia infiltration that could correspond to the clinical response. Thirty months after the resection of the lesion, the patient was still in complete remission.
Figure 3: Tumour response in patient #3 treated with Imatinib
A 34 year-old man treated for 4 months with Imatinib. We report here the radiological evolution of his DFSP comparing pre-Imatinib baseline status (left panel) and post-Imatinib status (right panel) before curative surgery. The MRI at baseline shows a thickening and heterogeneous hypersignal intensity of extra-cranial soft tissues of the left fronto-parietal calvarium (arrows) on coronal T2-weighted MR image with Fat Saturation. Thickness of tumoral process was about 7 mm and transversal extent about 5 cm. The Coronal contrast-enhanced CT scanner reformatted image in similar slice location as on MRI showing dramatic post-treatment tumor shrinkage (arrows). Complete recovery was considered because only a few aspecific but presumptively scaring tractus were seen within thinned sub-cutaneous tissues.
Patient 4
In October 2010, a 62 year-old woman presented at the dermatology consultation with a large skin lesion of her back with satellite tumour around the main one. These lumbar lesions were developing since 2005. A biopsy confirmed the diagnosis of DFSP and the baseline CT-scan showed axillary and inguinal lymph nodes, bilaterally. We decided to not perform a first intention lymph node biopsy but to start a neoadjuvant treatment with imatinib mesylate at a dose of 400mg/d. After 4 months of treatment, a partial response was observed with shrinkage of the lesion from 59x40x45mm to 45x21x41mm. The response was seen mainly within the first weeks of treatment. Due to the presence of suspect lymph nodes on the baseline CT-scan (but no histologic proof of malignity), an axillary and inguinal node evaluation was achieved during the same time of the tumour resection according to the sentinel lymph node technique. A complete resection of the skin lesion was performed with 3 cm macroscopic free margin in order to remove the satellite lesion using the same incision. We have combined this resection with the excision of 2 lymph nodes in the right inguinal area, 6 in the right and 1 in the left axillary area, based on the lymphoscintigraphic mapping. The pathological analysis described a large dermatofibrosarcoma measuring 90x55mm corresponding to the main tumour along with the satellite lesion. The lateral margins were safe with a minimum of 22mm and the deep margin show a subcutaneous fascia free of tumour infiltration. All the lymph nodes were free of tumour infiltration. The patient is still free of recurrence 19 months after the surgery.
Discussion
DFSP is a rare skin sarcoma featured by an indolent slow dermal growth of which main treatment option is surgery. Controversies exist about the kind of surgery to be performed: either wide margins of at least 3 cm or Mohs micrographic surgery [14]. Large free margins remain a cornerstone of DFSP treatment since they are correlated with a better disease-free survival. Following the localization and/or the size of the primary lesion, the resection could lead to mutilating surgery with a long post-operative morbidity and/or cosmetic and functional impairments. Relapsing DFSP or metastatic DFSP could be treated by radiotherapy or chemotherapy. These palliative treatments are not associated with increase in overall survival. Few trials evaluating new drugs including imatinib mesylate have been performed in DFSP patients [15-17]. It is obviously impossible to perform large randomized trials in view to evaluate the efficiency of a new treatment because of the rarity of DFSP. The largest trial evaluating Imatinib as treatment for relapsing or metastatic DFSP has been reported in 2010 [15]. It was a pooled analysis of 25 patients included in the SWOG0345 and EORTC62027 trials. Each of these studies included 40 patients but were prematurely closed due to poor accrual. The response rate observed was 37.5% and no striking difference was observed between patients receiving imatinib mesylate at 400 mg/d or 800 mg/d [15]. Among those, 4 patients underwent a surgery to remove DFSP but no information was provided on the outcome of these patients. Based on this observation, we attempted to use imatinib mesylate as a neoadjuvant treatment for DFSP. This could be a good indication when the lesion is large or badly localized on the face, the upper or the lower extremity and thus far jeopardizing large free margins. In the fibrosarcomatous variant of DFSP, we have some clinical relevant data showing a benefit of a treatment with Imatinib when the tumour is harbouring a COL1A1-PDGFB transcript [18]. The treatment of large or locally advanced DFSP with neoadjuvant imatinib mesylate requires synergies within a multidisciplinary team including radiologist, dermatologist, anatomopathologist, medical oncologist and surgeon. This team must adequately select eligible patients for the combined treatment and follow them regularly to choose optimal scheduling for the resection.
In our study, we have treated 4 patients with initially unresectable DFSP with imatinib mesylate as neoadjuvant treatment. Three patients received the treatment during four months and one during ten months. In the first patient, we observed a good partial response based on RECIST criteria. We observed dramatic tumour shrinkage after imatinib mesylate treatment in patient 3 corresponding to a complete response according to RECIST. In the two other patients, we have observed stable disease based on RECIST since only a thinning in the soft tissue around the lesion suggesting a regression of the infiltration around the tumour. These two last cases also illustrate the limit of using RECIST in order to define response. In all cases, we observed a fibrotic proliferation after treatment associated with a significant decrease in the cellularity of DFSP (Figure 1, comparison of panels C and F). All the patients underwent a curative complete excision of their tumour with confirmation of free margins. No adjuvant treatment was given.
We have compared our results with those in the literature (Table 2). Only one multiple case-report of 4 patients and a small phase II trial with 25 patients have been published [1,3]. In the study of Kerob, the neoadjuvant treatment was given during two months with a response rate of 36%, without any information about the follow-up or the relapse free survival [3]. In the Hanâ??s study, they have a response rate of 100% but we don't know if it's accorded to the RECIST criteria. In all our patients, radiological modifications were observed and in two of them a true response according to the RECIST criteria was recorded. Our 4 patients underwent a complementary curative surgical procedure with free margins. Our observation is in line with the one of Han. The lower response rate observed in the phase II study could be due to the shorter treatment duration of 2 months [3]. Our first patient received imatinib mesylate for 10 months but the maximum reduction of the tumour was observed after 4 months. This observation guides us to propose a shorter treatment of 4 months to the subsequent patients. Of course, we need more data to conclude about the duration of the neoadjuvant treatment, this one could be currently guided case by case after clinical and radiological evaluations.
|
|
Number |
Treatment : Imatinib |
Surgical |
Relapse |
FUP |
||
|
Dose (mg/d) |
Duration |
Response Rate |
|||||
|
HAN |
4 |
600 |
3-3.5-4-7 mos |
100 % |
mohâ??s |
0 % |
48mos |
|
KEROB |
25 |
600 |
2 mos |
36 % |
? |
? |
? |
|
PONCIN |
4 |
400 |
4-4-4-10 mos |
50 % |
minimal |
0 % |
32mos |
Table 2: Published reports on neoadjuvant treatment of DSFP with Imatinib
Abbreviation: FUP, median follow-up.
In both published studies, the dose of imatinib mesylate was 600 mg/d which is higher that has been reported for the treatment of this disease. At 400mg/d we didnâ??t observed severe toxicity. Only one patient presented with a grade 1 fatigue and grade 1 nausea. No haematological toxicity or arthromyalgia was observed and no dose reduction was needed. We propose a dose of 400mg/d in the neoadjuvant setting. In fact, in the largest study evaluating Imatinib in the metastatic or unresectable dermatofibrosarcoma, we did not observed any meaningful difference between the dose of 400 and 800mg/d [15].
All of our patients are still in complete remission after a median follow-up of 32 months. The disease-free survival of the 4 patients is 71, 34, 30 and 19 months, respectively. For the patient 2 and patient 4, we obtained small free margins of only 1 mm but none of these two patients relapse at the time of the last follow-up evaluation. This could suggest that margins could be downsized after neoadjuvant treatment with imatinib mesylate in DFSP, which results in significant benefits on a cosmetic and functional point of views, particularly in young patients. All these hypothesis need to be validated in a phase II trial with a minimal follow-up of three years according to the main time of recurrence in the old series with minimal surgery.
References
- Han A, Chen EH, Niedt G, Sherman W, Ratner D (2009) Neoadjuvant imatinib therapy for dermatofibrosacroma protuberans. Arch Dematol 7: 792-796.
- Enzinger FM, Weiss SW (1988) Fibrohistiocytic tumors of intermediate malignancy. In: Stamatis, G. Soft Tissue Tumors. Mosby, St Louis, MO 252.
- Kerob D, Porcher R, Vérola O, Dalle S, Maubec E, et al. (2010) Imatinib mesylate as a preoperative therapy in dermatofibrosacroma: results of a multicenter phase II study on 25 patients. Clin Cancer Res16: 3288-3295.
- Taylor HB, Helwig EB (1962) Dermatofibrosarcoma protuberans. A study of 115 cases. Cancer 15: 717-725.
- Haycox CL, Odland PB, Olbricht SM, Piepkorn M (1997) Immunohistochemical characterization of dermatofibrosarcoma protuberans with practical applications for diagnosis and treatment.J Am Acad Dermatol37: 438-444.
- Gloster HM Jr (1996) Dermatofibrosarcoma protuberans. J Am Acad Dermatol35: 355-374.
- Nickoloff BJ (1991) The human progenitor cell antigen (CD34) is localized on endothelial cells, dermal dendritic cells, and perifollicular cells in formalin-fixed normal skin, and on proliferating endothelial cells and stromal spindle-shaped cells in kaposi's sarcoma.Arch Dermatol 127: 523-529.
- Dominguez-Malagon HR, Ordonez NG, Mackay B (1995) Dermatofibrosarcoma protuberans: ultrastructural and immunocytochemical observations. Ultrastruct Pathol 19: 281-289.
- Stacchiotti S, Pedetour F, Negri T, Conca E, Marrari A, et al. (2011) Dermatofibrosarcoma protuberans-derived fibrosarcoma : clinical history, biological profile and sensitivity to imatinib. Int J Cancer7: 1761-1772.
- Fields RC, Hameed M, Qin LX, Moraco N, Jia X, et al. (2011) Dermatofibrosarcoma protuberans (DFSP): predictors of recurrence and the use of systemic therapy. Ann Surg Oncol18: 328-336.
- Muchemwa FC, Wakasugi S, Honda Y, Ihn H (2009) PDGFB quantification is a useful tool in the diagnosis of dermatofibrosarcoma protuberans: a study of 10 cases. Clin Exp Dermatol35: 295-299.
- Sjöblom T, Shimizu A, O'Brien KP, Pietras K, Dal Cin P, et al. (2001) Growth inhibition of dermatofibrosarcoma protuberans tumors by the platelet-derived growth factor receptor antagonist STI571 through induction of apoptosis. Cancer Res 61: 5778-5783.
- Rubin BP, Schuetze SM, Eary JF, Norwood TH, Mirza S, et al. (2002) Molecular targeting of platelet-derived growth factor B by imatinib mesylate in a patient with metastatic dermatofibrosarcoma protuberans. J Clin Oncol 20: 3586-3591.
- Martin RN, Acland KM, Williams HC (2012) Is Mohs micrographic surgery more effective than wide local excision for treatment of dermatofibrosarcoma protuberans in reducing risk of local recurrence? A Critically Appraised Topic. Br J Dermatol 167: 6-9.
- Rutkowski P, Van Glabbeke M, Rankin CJ, Ruka W, Rubin BP, et al. (2010) Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials.J Clin Oncol10: 1772-1779.
- Rutkowski P, Debiec-Rychter M, Nowecki Z, Michej W, Symonides M, et al. (2011) Treatment of advanced dermatofibrosarcoma protuberans with imatinib mesylate with or without surgical resection. J Eur Acad Dermatol Venereol3: 264-270.
- Malhotra B, Schuetze SM (2012) Dermatofibrosarcoma protuberans treatment with platelet-derived growth factor receptor inhibitor: a review of clinical trials results. Curr Opin Oncol24: 419-424.
- Kerob D, Pedeutour F, Leboeuf C, Verola O, de Kerviler E, et al. (2008) Value of cytogenetic analysis in the treatment of dermatofibrosarcoma protuberans. J Clin Oncol 26: 1757-1759.