Editorial
Authors:
DR. G Ramasubrahmanyam, MS, MCh*1
1Chief Consultant Cardiothoracic Surgeon, Care Hospitals, India
Corresponding author
G Ramasubrahmanyam, Chief Consultant Cardiothoracic Surgeon, Care Hospitals, Road Number 1, Banjara Hills, Hyderabad, Telangana, India, Tel: +91 9849021975, E-mail: d_gutti@rediffmail.com
Received Date: 06 July 2017; Accepted Date: 06 July 2017; Published Date: 16 July 2017
Citation
Ramasubrahmanyam G (2017) The Role of Induction Therapy in Heart Transplant. Is it Obligatory? Enliven: Surg Transplant 4(e2): e002.
Copyright
@ 2017 Dr. G Ramasubrahmanyam. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License that permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Rejection is the major cause of early morbidity and mortality post heart transplantation. Immunologic mechanisms are responsible for causing graft rejection, which can occur in various ways. The rejection is primarily a T-lymphocyte (T cell) mediated event, although humoral (B-cell) responses also contribute. Hyperacute rejection occurs when preformed antibodies to human leukocyte antigens (HLA) develop resulting in an immediate rejection. The recognition of donor antigens begins with the function of antigen presenting cells (APCs). Donor APCs are expressed as donor alloantigens which are directly recognized by recipient T-cells which is known as direct allorecognition. When the donor alloantigens are shed by the graft which are taken up by the recipient APCs and then presented to T-cells, which is known as indirect allorecognition. These alloantigens are recognized by the T-cell receptor (TCR) CD-3 complex on the surface of T-cell. But the levels of T-cell activation to cause a rejection occurs only when there is a second or a co-stimulatory signal between APC and T-cell. There are several co-stimulatory molecules that function as receptor ligand pairs on APC and T-cell surface. Engagement of the TCR-CD3 complex, followed by co-stimulatory signals, results in activation of calcineurin in the cytoplasm of the T-cell. The nuclear factor of activated T-cell (NF-AT) is transcribed by the dephosphorylated calcineurin which then binds to interleukin 2 (IL-2) in the nucleus which activates the cell surface IL-2 receptor. This stimulates clonal expansion of T helper cells, cytotoxic T cells, B cells and natural killer cells. Engagement of the IL-2R activates the enzyme target of rapamycin (TOR). TOR regulates the translation of mRNAs to proteins
History
The first solid organ to be transplanted was a cadaveric kidney in 1933. The immunosuppression used was total body radiation which was unsuccessful. In 1960, 6-mercaptopurine and azathioprine, considered as breakthrough drugs, had become the standard of care in pharmacological immunosuppression. In the year 1960, Richard Lower and Norman Shumway reported the survival of up to 3 weeks in dogs that were given a heart transplant, at the University of Stanford. The development of donor and recipient matching in 1962, in addition to the immunosuppressive regimens, helped in performing successful organ transplants for the next 20 years. In 1964, the first heart to be transplanted to a human using a chimpanzee donor heart was James Hardy. In 1967, Christian Bernard performed the human to human heart transplantation, who died on the 18th post-operative day. The first anti-thymocyte globulin was developed in 1967 followed by subsequent development of monoclonal and polyclonal antibodies. Philip Caves and associates introduced percutaneous endomyocardial biopsy that allowed the early diagnosis and treatment of acute rejection. The development of cyclosporine in 1980, a calcineurin inhibitor was a crucial development in successful heart transplantation. Immunosuppressive drugs can be classified as induction therapies, maintenance therapies and anti-rejection therapies [1]. In the current era of heart transplantation, induction of immunosuppression after a heart transplant has been very crucial in determining the outcomes and hence being studied meticulously.
Introduction
Rejection is the major cause of early morbidity and mortality post heart transplantation. Immunologic mechanisms are responsible for causing graft rejection, which can occur in various ways. The rejection is primarily a T-lymphocyte (T cell) mediated event, although humoral (B-cell) responses also contribute. Hyperacute rejection occurs when preformed antibodies to human leukocyte antigens (HLA) develop resulting in an immediate rejection. The recognition of donor antigens begins with the function of antigen presenting cells (APCs). Donor APCs are expressed as donor alloantigens which are directly recognized by recipient T-cells which is known as direct allorecognition. When the donor alloantigens are shed by the graft which are taken up by the recipient APCs and then presented to T-cells, which is known as indirect allorecognition. These alloantigens are recognized by the T-cell receptor (TCR) CD-3 complex on the surface of T-cell. But the levels of T-cell activation to cause a rejection occurs only when there is a second or a co-stimulatory signal between APC and T-cell. There are several co-stimulatory molecules that function as receptor ligand pairs on APC and T-cell surface. Engagement of the TCR-CD3 complex, followed by co-stimulatory signals, results in activation of calcineurin in the cytoplasm of the T-cell. The nuclear factor of activated T-cell (NF-AT) is transcribed by the dephosphorylated calcineurin which then binds to interleukin 2 (IL-2) in the nucleus which activates the cell surface IL-2 receptor. This stimulates clonal expansion of T helper cells, cytotoxic T cells, B cells and natural killer cells. Engagement of the IL-2R activates the enzyme target of rapamycin (TOR). TOR regulates the translation of mRNAs to proteins [2].
Rejection is classified as hyperacute, acute cellular and acute humoral (vascular) or chronic. Hyperacute occurs within minutes to hours. It is caused by preformed antibodies to ABO blood group antigens, HLA or endothelial antigens. But with improved matching of recipients and donors, hyperacute rejection is rare now. Acute cellular rejection may occur anytime in first 3 to 6 months. It is a T cell mediated response with infiltration of lymphocytes and macrophages and resultant myocytolysis. Acute humoral rejection occurs days to weeks after heart transplantation and is initiated by antibodies rather than T cells.
Although induction therapy has been used in heart transplantation for many years, its role has not been fully elucidated. Induction therapy has been part of the immunosuppressive armamentarium for more than 40 years, but there is still uncertainty about when and how it should be used in heart transplantation. Early safety concerns arising from the administration of OKT3 or high-dose lymphocyte-depleting regimens, particularly regarding infectious complications, have largely been overcome. Induction immunosuppression if initiated early after a heart transplant reduces the risk of acute graft rejection. But induction of immunosuppression is only used by approximately 50% of centers as per the international society for heart and lung transplantation. The agents used for induction of immunosuppression are an interleukin -2 receptor antagonist, a polyclonal antithymocytic (ATG) or an anti-lymphocytic (ALG) antigen and OKT 3 [3].
Anti-HLA antibodies can be detected before transplantation using different techniques. Complement-dependent lymphocytotoxicity assays are widely used for measurement of panel reactive antibody (PRA) and for crossmatch purposes. In order to determine whether or not a patient already has any specific HLA antibodies, a lab specialist will test a patient’s blood (serum) against lymphocytes (white blood cells) obtained from a panel of about 100 blood donors. These 100 donors represent the potential HLA makeup for a donor from that area. Percent PRA (%PRA) is the number of reactions within that panel. If a candidate’s serum does not react with any of the donor samples, the candidate is not sensitized and has a PRA of 0. If a candidate’s serum reacts in 80 out of 100 samples, the patient has a PRA of 80%. Theoretically, that means that if a donor becomes available from that donor pool, the recipient would experience acute rejection 8 out of 10 times. That patient might have to wait a very long time until a compatible donor becomes available. Newer assays using solid-phase flow techniques feature improved specificity and offer detailed information concerning antibody specificities, which may lead to improvements in donor-recipient matching. Plasmapheresis, intravenous immunoglobulin, and rituximab have been used to decrease the PRA before transplantation, with varying degrees of success.
Monoclonal and polyclonal antithymoglobulins are used in most centers and considered as optimal inducing agents. Rabbit antithymocyte globulin (rATG) has been utilized for adult and pediatric heart transplant recipients since 30 years. Two types of rATG are commercially available: Thymoglobulin, ATGAM and ATG-Neovii.
Induction Therapy Regimen
The induction regimen for rATG post heart transplant as per clinical trials before the year 2000 was 10.5 to 15 mg/kg as a total dosage. Later trials showed the dosage did not exceed 7.5 mg/kg. For Thymoglobulin the license states the dosage range is between 3 mg/kg to 12.5 mg/kg, given over 3 to 5 days. For ATG Neovii the dosage range is 2 to 5 mg/kg over a period of 5 to 14 days. The lymphocyte depletion in post-transplant ATG therapy is significantly higher with lower doses (total; 7.5 mg/kg) between 7 to 21 days than a higher dosage of 10.5 mg/kg total. Higher doses cause a very significant immunosuppression with a very high risk for fatal infections and malignancy [4]. Earlier when CMV vaccinations were not used widely, it was a common cause of infection in the early post heart transplant period, which is uncommon in the present era. The comparison of ATG vs basiliximab showed incidence of lesser infection with the use of latter. But basiliximab and dacliuzumab are no longer used in heart transplant patients.
Timing and Dosage of rATG
The first dose in given within 1 to 2 hours of receiving the patient in the ICU and confirming that there is no bleeding and is hemodynamically stable. In case of right heart failure and pulmonary hypertension, the rATG can be delayed. If patients are unresponsive to the treatment for right heart failure within 24 hours, rATG can be initiated at 1.5 mg/kg/day. In patients with a potential risk of primary graft failure, it is favorable to initiate a 12 hour infusion either before the transplant or after the initiation of anesthesia. For all other patients the standard total dose range is 4.5 to 7.5 mg/kg, to be given over 3 to 5 days, can be prolonged if the dosage is lowered. In patients with slightly lower immunological risk, 3.0 to 4.5 mg/kg dose can be used. In patients with higher immunological risk the total rATG dose should be >4.5 mg/kg, and 6.0 – 7.5 mg/kg being appropriate. In those with pre-transplant mechanical circulatory support, a dose between 1.5 and 2.5 mg/kg is considered. The duration of rATG infusion should be
Over 8-12 hours (Table-1). Adverse effects of rATG are allergic reaction (fever, hypotension), thrombocytopenia. These along with signs of sepsis are indications to stop the infusion. rATG dose should be reduces when platelet count <75,000 and stopped when count is <50,000. The premedication for rATG initiation are H1 and H2 blockers, steroids and antipyretic therapy [4].
|
Medication |
Dose |
Duration |
|
Corticosteroids |
||
|
Methylprednisolone(high dose) |
250-1000 mg/day IV |
3 days |
|
prednisolone |
1-3 mg/kg/day PO |
3-5 days |
|
Polyclonal anti-thymocyte antibody |
||
|
Thymoglobulin |
0.75-1.5 mg/kg/day |
5-14 days |
|
ATGAM |
10 mg/kg/day |
5-14 days |
|
ATG-Fresenius |
3 mg/kg/day |
5-14 days |
|
Monoclonal antibody |
||
|
Muromonab-CD3 (OKT-3) |
5 mg/day |
5-14 days |
|
IL-2 receptor antibodies |
||
|
Basiliximab |
12 mg/m2, upto 20 mg per dose |
Day 0 & day 4 |
|
Daclizumab |
1 mg/kg IV |
Perioperatively, later every 2 weeks (5 doses) |
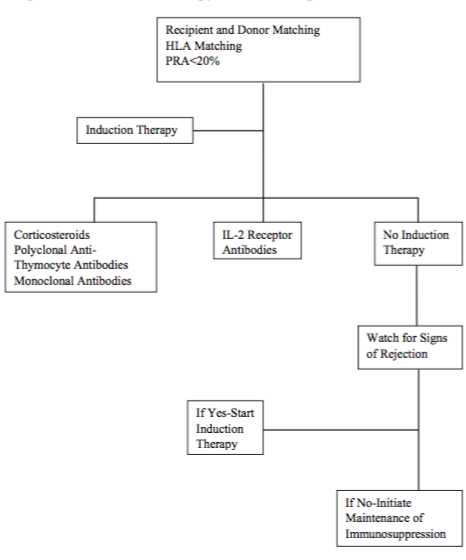
Figure 1:Algorithm for Induction therapy for heart transplantation at our center.
When to Use CNI
This is mainly dependent on the patient’s renal function. It cannot be initiated if the GFR is < 50-60 ml/hour. With a reduction in serum creatinine, CNI can be started on 3rd post-operative day. But with CKD, it can be delayed from 5 to 10 days, not later than this. For standard or low immunological risk, 6-10 ng/ml for Tacrolimus and 100-200 ng/ml for Cyclosporine A (CsA) should be considered. The Schedule study indicates that switching from CNI therapy to Everolimus at 7-11 weeks post-transplant can improve preservation of renal function. For those with high immunological risk, Tacrolimus (10-15 ng/ml) is preferred over CsA (200-300 ng/ml) [4]. The pre-transplant use of antibiotics should be continued for 10-14 days after transplant. The rATG dose and the CNI should not overlap and also there should not be any gap between the end of rATG dosing and initiation of CNI. A gap of 3 to 4 days can be considered only in those with a high immunological risk.
The Indian Scenario
In Indian scenario, where cost of therapy is a major constraint and with the present experience and evidence, we can avoid induction therapy. With further experience, we can formulate our own strategies for avoiding anti-rejection medication and balancing the risk of infection. In our view, a meticulous pre-transplantation work-up beginning with the recipient and donor in terms of PRA testing, donor and recipient cross-matching and if possible HLA matching are very important for outcomes and measures, for not to use induction therapy. In our place, majority of patient population has cost affordability issues, which led us to a thorough pre-transplant work-up with proper donor selection, so that graft rejection is minimized. But, one needs to be very cautious and prepared for any kind of an event which give out early signs of rejection, where the induction therapy should be initiated without delay.
Conclusion
Heart transplantations are on the rise as the patient population are getting aware of it and also the widely expanding health sector availing its services far and wide. We advise for a good planning with a trained team to start the pre-transplant evaluation with a view to avoid rejection as much as possible and keep the induction therapy only as a standby. It needs a very efficient team of cardiologists, cardiac surgeons, paramedics, nursing staff and other logistics personnel who are trained and can carry out the mammoth task with ease. Very soon we might be able to perform heart transplants without the use of induction therapy in majority of our patients with uncompromised results.
References
- Jeffrey B. Velotta, Leora B. Balsam, Michael P. Fischbein, and Robert C. Robbins. Chapter on heart transplant, Sabistan and Spencer surgery of the chest. Page number – 1533-1534.
- Hans Lehmkuhl, Michael Dandel, Nicola Hiemann, Christoph Knosalla, Miralem Pasic, et al. (2011) Induction therapy in heart transplantation. Applied Cardiopulmonary Pathophysiology 15: 241-244
- Aliabadi A, Grommer M, Cochrane A, Salameh O, Zuckermann A (2013) Induction therapy in heart transplantation: where are we now? Transpl Int 26: 684-695.
- Barten MJ, Schulz U, Beiras-Fernandez A, Berchtold-Herz M, Boeken U, et al. (2016) A Proposal for Early Dosing Regimens in Heart Transplant Patients Receiving Thymoglobulin and Calcineurin Inhibition. Transplant Direct 2: e81.