Research Article
LU Peng*, LIU Lijun, and WANG Jian
*College of Biological and Agricultural Engineering, Jilin University, Changchun 130022, P.R. of China
Corresponding author
LU Peng, College of Biological and Agricultural Engineering, Jilin University, Changchun 130022, P.R. of China, E-mail: lupeng13@mails.jlu.edu.cn
Received Date: 25thAugust 2015
Accepted Date: 28sth September 2015
Published Date: 02nd October 2015
Citation
Peng L, Lijun L, Wang J (2015) The Optimization of L-Isoleucine Fermentation Conditions by Brevibacterium flavum KM011. Enliven: Microb Microbial Tech 1(2): 002.
Copyright
2015 Dr. LU Peng. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
In order to improve the production of L-isoleucine, the seed culture medium and the fermentation culture medium were examined by L-isoleucine
producing strain Brevibacterium flavum KM011(Met-+Ethr+α-ABr+LysL+AECr). Under the optimizing seed culture medium and fermentation culture
medium, L-isoleucine productions were increased to 9.493 g/L and 13.35 g/L in shake flasks, respectively. The strain KM011 got a final titer of 35.26
g/L under the optimal conditions by using 5L fermenter.
Keywords
L-isoleucine; Fermentation; Culture medium optimization
Introduction
L-isoleucine is one of the eight essential amino acids and belongs to branched chain amino acid [1]. It helps repairing muscles, controlling blood sugar and providing energy to the body tissues [2].
Fermentation culture medium components and feeding mode are main factors influencing the yield of L-isoleucine. Several carbon sources have been tested to find the most efficient carbon supplier, and glucose was proved to be appropriate [1]. Inoculum concertration, pH controlling methods, dissolved oxygen have been tested; the optimal conditions have been obtained. The results showed that 10% inoculums concentration, pH controlled by CaCO3 added for 2% (w) and 24% (w) urea added by batch and the volume of liquid in 35ml could get the optimal fermentation production separately [2]. Using MATLAB software package, the optimal medium proportion was attained for glucose 16g/dl, (NH4)2SO4 1.25 g/dl, VH 14μg/dl, VB1 0.1mg/dl [1]. Glucose, ammonium sulfate, potassium dihydrogen phosphate, leucine and corn syrup have been found to be the most effective components in fermentation culture medium, and the optimal proportion was obtained by uniform design. The results showed the L-isoleucine production was 21.3g/L with glucose 120, (NH4)2SO4 50, KH2PO4•3H2O 2, L-leucine 0.1 and corn syrup 15(g/L) [3]. DO-stat feeding strategy was adopted to increase L-isoleucine production [4]. The result demonstrated that when DO was controlled at 20%, L-isoleucine production would increase to 24.3g/L. With dual exponential feeding and two-stage dissolved oxygen control strategy, the L-isoleucine production increased to and 31.32g/L after 60h [5].
Betaine is not only a widely known osmolyte [6] but also a methyl donor as well as methionine, choline, folate [7]. Vitamin B12 (VB12), also called cobalamin, can catalyzes the transfer of methyl group from N5-methyltetrahydrofolate to homocysteine [8], offering a methyl donor way. And methionine is methyl donor, too. Pantothenic acid (VB5) is precursor substance of Coenzyme A(CoA). CoA is formed by a universal series of reactions beginning with the phosphorylation of pantothenic acid [9]. With pantothenic addition, Proteus morganii show a little decomposition and slightly oxydation of L-isoleucine [10].
In this study, betaine, VB12 and VB5 were added separately into seed and fermentation culture medium to find their optimal additional concentration.
Materials and Methods
Microoganisms
Brevibacterium flavum KM011 (Met-+Ethr+α-ABr+LysL+AECr) was stored at fermentation engineering laboratory of Jilin University.
Culture media and growth conditions
Activated slant medium (glucose 0.1%, beef extract 1%, peptone 1%, yeast powder 0.5%, NaCl 0.25%, agar 1.5%, PH 7.0) was used for inoculums preparation. The seed medium contained the following (in g/L): glucose 35, ammonium sulfate 3, KH2PO4•3H2O 1.5, MgSO4•7H2O 0.4, FeSO4•7H2O 0.01, MnSO4•H2O 0.01, soybean cake hydrolysate 15 mL, corn syrup 15 mL, VH 0.5 mg, VB1 2.5 mg, urea 2.0, pH 7.0. The medium for L-isoleucine fermentation contained the following (in g/L): glucose 80, (NH4)2SO4 15, FeSO4•7H2O 15mg, MgSO4•7H2O 0.4, MnSO4•H2O 15 mg, KH2PO4 1.5, K2HPO4 3.0, VH 140 μg, VB1 0.1 mg, Met 20 mg, soybean cake hydrolysate 20mL, corn syrup 25mL, CaCO3 20. The pH of both seed and fermentation medium was adjusted to 7.0 with 4M NaOH. A 500ml conical flask containing 50ml seed medium was inoculated with strain KM011 and cultivated at 31.5°C, 200r/min for 20 h. Fed-batch fermentation was performed in 5L fermenter. 5L fermenter containing 2.55L production medium were inoculated aseptically with culture developed in the conical flask (15% v/v). In the fermentation process of L-isoleucine, the temperature was maintained at 31.5°C and the pH was adjusted to 7.0 with concentrated ammonia. The DO level was maintained at approximately 20% saturation by adjusting the agitation and aeration rates. When the initial glucose was depleted, 800 g/L glucose solution was fed into the fermenter to meet specific experimental requirements.
Optimization for Concentration of Additives
To get the maximum yield of L-isoleucine under different additional backgrounds, betaine, VB12 and VB5 were added separately in seed and fermentation culture medium with appropriate concentration. The results were tested in shake flask and 5L fermentation tank. Certain amounts of fermentation liquids were taken every 3h for measuring the residual sugar, pH and L-isoleucine production in shake flask. The pH was controlled to 7.0 by adding concentrated ammonia. Certain amounts of fermentation liquids were taken every 4h for measuring the residual sugar, cell concentration (expressed by absorbency) and L-isoleucine production in 5L fermentation tank.
Analysis of Fermentation Products
Determination of residual sugar was performed by SBA-40E Bio sensing analyzer. Absorbency was detected by using UV-2000 spectrophotometer to measure the optical density at the wavelength of 560nm, diluted 50 times, path 1cm. Determination for the content of L-isoleucine was performed by paper chromatography [11].
Results and Discussions
Optimization of Seed Culture Medium
Optimization of betaine concentration
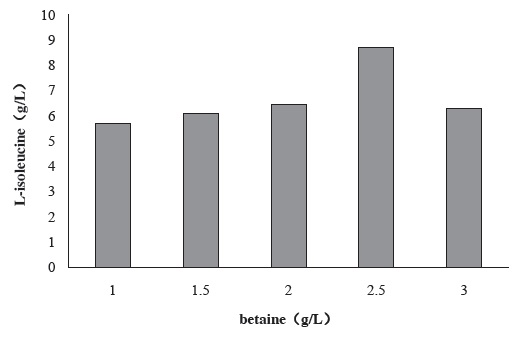
Figure 1 shows the effect of betaine concentration on seed culture medium for the yield of L-isoleucine by strain KM011. Betaine can inhibit permeate exude from cell and reduce the energy waste [12,13]. With it, strains can hold cell morphology under osmotic pressure. Also, betaine is methyl donor as well as methionine. Strain KM011 is a methionine deficiency, so adding betaine in seed culture medium is an efficient way to enhance the growth of strains.
Figure 1 Effect of betaine concentration in seed culture medium on the production of L-isoleucine by strain KM011
Under the condition of 2.5 g/L betaine addition, L-isoleucine production has been enhanced to 8.71 g/L.
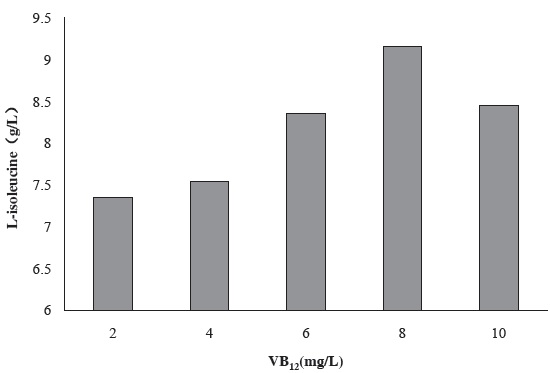
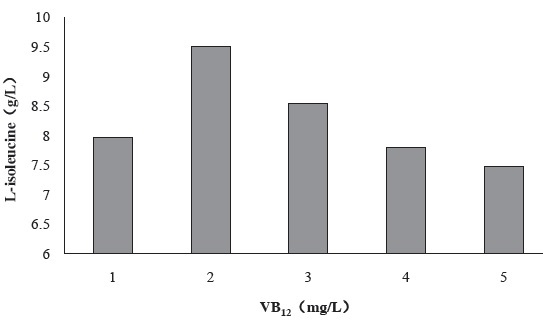
Optimization of VB12 concentration
Figure 2 shows the effect of VB12 concentration in seed culture medium on the production of L-isoleucine by strain KM011. VB12 is dependent for methionine synthase; it can catalyzes the transfer of methyl group from N5-methyltetrahydrofolate to homocysteine, producing tetrahydrofolate and methionine. Strain KM011 is methionine deficiency, so adding VB12 in seed culture medium is an efficient way to enhance the growth of strains. By the way, the period has been shortened from 20h to 12h by seed culture and from 48h to 37h by fermentation culture.
Under the condition of 8 mg/L VB12 addition, L-isoleucine production has been enhanced to 9.16 g/L.
Figure 2 Effect of VB12 concentration in seed culture medium on the production of L-isoleucine by strain KM011
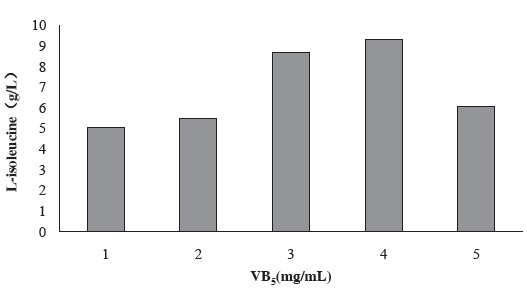
Optimization of VB5 concentration
Figure 3 shows the effect of VB5 concentration in seed culture medium on the production of L-isoleucine by strain KM011. VB5 is precursor substance of CoA which is essential substances for energy metabolism from sugar and fat. VB5 can also enhance the growth of strains.
Under the condition of 4 mg/L VB5 addition, L-isoleucine production has been enhanced to 9.32 g/L.
Figure 3 Effect of VB5 of different concentration in seed culture medium for the production of L-isoleucine by strain KM011
Optimization of Fermentation Culture Medium
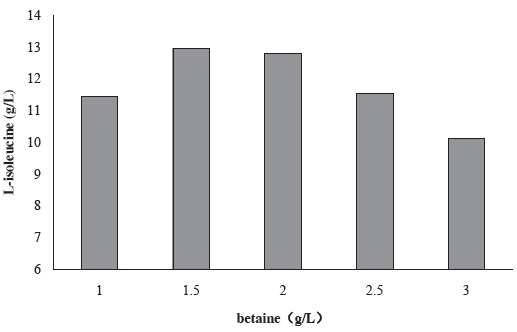
Optimization of betaine concentration
Figure 4 shows the effect of betaine concentration in fermentation culture medium on the production of L-isoleucine by strain KM011. Betaine can inhibit permeate exude from cell and reduce the energy waste. That means for L-isoleucine producing strain KM011, betaine could promote the excess of L-isoleucine efflux, thereby reducing the feedback inhibition.
When 1.5 g/L betaine was added to the culture medium, L-isoleucine production was enhanced to 12.94 g/L.
Figure 4 Effect of betaine concentration in fermentation culture medium on the production of L-isoleucine by strain KM011
Optimization of VB12 concentration
Figure 5 shows the effect of VB12 concentration in fermentation culture medium on the production of L-isoleucine by strain KM011. VB12 can catalyze the formation of methionine, which is a well known methyl donor. Strain KM011 is methionine deficiency, so VB12 probably can act as a methyl donor. Under the condition of 2 mg/L VB12 addition, L-isoleucine production has been enhanced to 9.51 g/L.
Figure 5 Effect of VB12 concentration in fermentation culture medium on the production of L-isoleucine by strain KM011
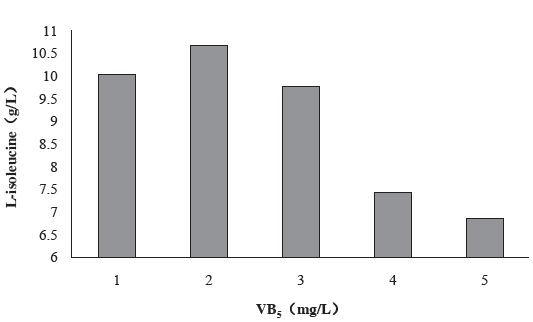
Optimization of VB5 concentration
Figure 6 shows the effect of VB5 concentration in fermentation culture medium on the production of L-isoleucine by strain KM011. VB5 is precursor substance of CoA. And CoA is essential substances in the conversion of carbohydrates and fats in energy. The addition of VB5 caused an improvement of metabolism in strain KM011, while the growth of the strain has been enhanced as a result.
Under the condition of 2 mg/L VB5 addition, L-isoleucine production has been enhanced to 10.67 g/L.
Figure 6 Effect of VB5 concentration in fermentation culture medium on the production of L-isoleucine by strain KM011
The Fed-Batch Fermentation for L-Isoleucine in 5L Fermenter
The experiment for L-isoleucine producing strain KM011 has been enlarged in 5L fermenter. By controlling pH, loading fluid volume, speed and dissolved oxygen more accurately, fermenter offered an optimal state of fermentation than shake flask. As a result, the production of L-isoleucine has been obviously improved in 5L fermenter. It is convenient using the fermenter for on-line and off-line detection; the experimental results can be regarded as guidance for genetic transformation of the species.
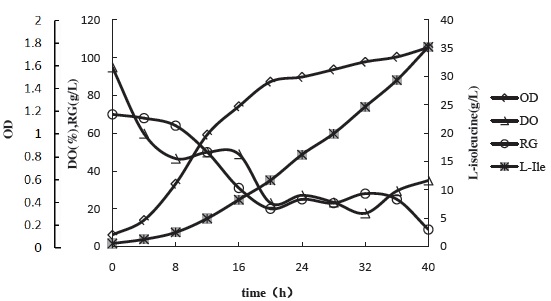
During the fermentation, certain amounts of fermentation liquid were taken every 4h for measuring the residual sugar, cell concentration (expressed by absorbency) and L-isoleucine production. Figure 7 shows the curve for fermentation process.
Figure 7 Curve for fermentation process of strain KM011
concentration during fermentation process
Whether it is too high or too low of cell concentration leads to a reducing of L-isoleucine production. When the cell concentration is low, the number of bacteria in the fermentation liquid is low, so the production couldn’t be high.
And when the cell concentration is high, the substrate consumption will increase. Substrates are mainly used for the synthesis of bacteria, so the production of L-isoleucine will reduce for substance competition. Meanwhile, the oxygen consumption will increase and the byproduct organic acid will accumulate, cause the inhibition of the L-isoleucine synthesis.
Figure 7 shows that the lag phase is short of the strain KM011. When it comes to the stable period, the amount of the strain KM011 is quite large. The strains enter the logarithmic growth phase by the 4th hour since the fermentation began. The growth of the strain keeps a high level, and the cell concentration keeps increasing all over the fermentation process.
Glucose concentration during fermentation process
The concentration of glucose in fermenter can be regulated by flow plusing 80% glucose. That is the way to provide essential nutrients, sustain the fermentation reaction.
Figure 7 shows that strain KM011 has a high glucose consumption rate. Since the fermentation began till the 16thh, the concentration of glucose decreased from 70 g/L to 30 g/L, the average glucose consumption rate was 2.5 g•L-1•h-1; The glucose supplement started at 16thh and the total supplementary glucose was 240 g/L, the fermentation period lasted for 40h, the average glucose consumption rate for the whole fermentation period was 6 g•L-1•h-1.
Dissolved oxygen during fermentation process
Aerobic fermentation also needs a dissolved oxygen limit. High level of dissolved oxygen surely will contribute to the growth of strain, but sometimes will inhibit the yield of product. Larger the amount of the bacteria, better the activity of the strains, so the oxygen consumption will increase by plus. The fluctuation of dissolved oxygen during the fermentation period will reflect the activity of strains.
Refer to Figure 7, in the early stage of fermentation period, the bacteria was in the delay period and the early logarithmic phase, the dissolved oxygen decreased gradually. To control the dissolved oxygen, speed, ventilation and tank pressure during the whole fermentation period were operated accurately. In the early period of fermentation, the ventilation and tank pressure were controlled to constant level, and dissolved oxygen was improved by increasing speed. When the speed came to 500-600 r/min, speed, ventilation and tank pressure were controlled alternately to stabilize the dissolved oxygen level. When it came to stationary phase, the concentration of the bacteria became large, the metabolism of the strains became exuberant and the speed, ventilation and tank pressure were getting the maximum level the experiment could ever supply. The determinant of the dissolved oxygen was the oxygen consuming ability of the bacteria. Figure 7 shows the large amount of the bacteria and the high oxygen consuming ability of the strain, the dissolved oxygen maintained to 20% for a while.
During the later stage of fermentation period, the strains became senescence, the activity of strains decreased, the oxygen consumption got low, and the dissolved oxygen increased.
L-isoleucine accumulation during fermentation process
Figure 7 shows that the accumulation of L-isoleucine lasted till the end of the fermentation. The strain KM011 could accumulate L-isoleucine of 35.26 g/L in 5L fermenter within 40h.
References
- Wenjun S, Ning C, Chun W, Shuyun L, Kexu Z (2003) Breeding of L-isoleucine Producer and Its Conditions Optimization on Fermentation Process. Food and Fermentation Industries 29: 34-37.
- Fei W, Ning C, Wen-jun S, Shu-yun L, Ke-xu Z (2004) Studies on the fermentation conditions of L-isoleucine. Journal of Shenyang Pharmaceutical University 21: 65-69.
- Jin L, Wei-guo Z (2006) Breeding of L-Isoleucine Producer and its Optimal Fermentation Conditions. Journal of Food Science and Biotechnology 25: 54-59.
- Junli Z, Long L, Jun F, Jianghua L, Guocheng D, et al. (2011) Optimization of L-isoleucine Fermentation Conditions Based on DO-stat Feeding Culture Strategy by Brevibacterium lactofermentation. Chin J Appl Environ Biol 17: 317-320.
- Cheng-lin Z, Hui L, Bing W, Jian W, Guo-dong X, et al. (2014) Effect of dual exponential feeding and two-stage dissolved oxygen control strategy on L-isoleucine production by Corynebacterium glutamicu. Food and Fermentation Industries 40: 1-6.
- Misra PP, Kishore N (2012) Volumetric and calorimetric investigations of molecular interactions in some amino acids and peptides in the combined presence of surfactants and glycine betaine. J Chem Thermodynamics 54: 453-463.
- Mahmoudnia N, Madani Y (2012) Effect of betaine on performance and carcass composition of broiler chicken in warm weather-A review. Int J Agri Sci 2: 675-683.
- Banerjee RV, Matthews RG (1990) Cobalamin-dependent methionine synthase. FASEB J 4: 1450-1459.
- Jackowski S, Alix JH (1990) Cloning, Sequence, and Expression of the Pantothenate Permease(panF)Gene of Escherichia coli. J Bacteriol 172: 3842-3848.
- Kersey RC, Porter JR (1948) Pantothenic Acid and the Metabolism of Amino Acids by Bacteria. Proc Soc Exp Biol Med 69: 379-382.
- Xiang JJ, Rong-Xian XU, Fan YF (2009) Effect of folic acid,VB6 and VB12 on expression of vascular cell adhesion molecule-1 in cultured endothelial cells in vitro. Chinese Journal of Public Health 25: 987-988.
- Alfieri RR, Cavazzoni A, Petronini PG, Bonelli MA, Caccamo AE, et al. (2002) Compatible osmolytes modulates the responses of porcine endothelial cells to hypertonicity and protect them from apoptosis. The J Physiol 540: 499-508.
- Moeckel GW, Shadman R, Fogel JM, Sadrzadeh SM (2002) Organic osmolytes betaine, sorbitol and inositol are potent inhibitors of erythrocyte membrane ATPases. Life Sci 71: 2413-2424.