Original Article
Pedja Kovacevic1, 2* , Sasa Dragic1 , Zvezdana Rajkovaca2, Tijana Kovacevic3
1Medical Intensive care Unit, Clinical centre Banja Luka, Bosnia - Herzegovina
2Department for Physiology, Medical School Banja Luka, Bosnia - Herzegovina
3Department for hospital pharmacy, Clinical centre Banja Luka, Bosnia - Herzegovina
Corresponding author
Pedja Kovacevic, Filipa Kljajica Fice 49, Banja Luka 78000, Republika Srpska, BiH Tel: ++387 65 668 400; E-mail: HYPERLINK ?mailto:peko051@yahoo.com?peko051@yahoo.com
Received Date: 20 June 2014
Accepted Date: 05 August 2014
Published Date: 10 August 2014
Citation
Pedja K, Sasa D,Zvezdana Raj,Tijana K.(2014) The Correlation Between Nitric Oxide Levels and Spirometry in Dialyzed Patients Compared to Healthy Subjects. Enliven:Nephrol Renal Stud 1(1):002.
Copyright
@ 2014 Dr.Pedja Kovacevic. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Several studies demonstrated increase in plasma concentration of nitric oxide (NO) in dialyzed patients (hemodialysis - HD and peritoneal dialysis - CAPD) compared to healthy control subjects. However, the effects of NO on respiratory function in these patients are less known. The aim of this study was to determine the potential differences in spirometry related to the serum levels of NO.
The study included 28 patients (15 males, mean age 55.9 ±16, 2 years) with end stage renal diseases receiving regular HD, 23 patients (10 males, mean age 55.8 ±15,8 years) treated with CAPD without any cardiovascular and respiratory diseases, and 30 healthy volunteers (14 males, mean age 51.8 ±15,6 years) in control group. The values of spirometry parameters were recorded in all studied patients. Serum levels of NO were measured by Griess reaction.
In groups of patients who were treated with HD or CAPD we found statistically significant difference in values of most pulmonary function parameters between subjects with NO level lower than 9,5 μmol/L and subjects with NO higher than 9,5 μmol/L. In the control group there was no difference in pulmonary function parameters in correlation to NO levels. NO values in patients of both dialyzed groups were significantly higher compared to healthy subjects. Grater level of NO in dialyzed patients than in healthy subject is associated with lower parameters of lung function tests. This can be explained by progression of inflammation, pulmonary oedema also known as “uraemic lung”or/and the progression of pulmonary hypertension.
Pedja Kovacevic1, 2* , Sasa Dragic1 , Zvezdana Rajkovaca2, Tijana Kovacevic3
1Medical Intensive care Unit, Clinical centre Banja Luka, Bosnia - Herzegovina
2Department for Physiology, Medical School Banja Luka, Bosnia - Herzegovina
3Department for hospital pharmacy, Clinical centre Banja Luka, Bosnia - Herzegovina
Corresponding author
Pedja Kovacevic, Filipa Kljajica Fice 49, Banja Luka 78000, Republika Srpska, BiH Tel: ++387 65 668 400; E-mail: HYPERLINK ?mailto:peko051@yahoo.com?peko051@yahoo.com
Received Date: 20 June 2014
Accepted Date: 05 August 2014
Published Date: 10 August 2014
Citation
Pedja K, Sasa D,Zvezdana Raj,Tijana K.(2014) The Correlation Between Nitric Oxide Levels and Spirometry in Dialyzed Patients Compared to Healthy Subjects. Enliven:Nephrol Renal Stud 1(1):002.
Copyright
@ 2014 Dr.Pedja Kovacevic. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Several studies demonstrated increase in plasma concentration of nitric oxide (NO) in dialyzed patients (hemodialysis - HD and peritoneal dialysis - CAPD) compared to healthy control subjects. However, the effects of NO on respiratory function in these patients are less known. The aim of this study was to determine the potential differences in spirometry related to the serum levels of NO.
The study included 28 patients (15 males, mean age 55.9 ±16, 2 years) with end stage renal diseases receiving regular HD, 23 patients (10 males, mean age 55.8 ±15,8 years) treated with CAPD without any cardiovascular and respiratory diseases, and 30 healthy volunteers (14 males, mean age 51.8 ±15,6 years) in control group. The values of spirometry parameters were recorded in all studied patients. Serum levels of NO were measured by Griess reaction.
In groups of patients who were treated with HD or CAPD we found statistically significant difference in values of most pulmonary function parameters between subjects with NO level lower than 9,5 ?mol/L and subjects with NO higher than 9,5 ?mol/L. In the control group there was no difference in pulmonary function parameters in correlation to NO levels. NO values in patients of both dialyzed groups were significantly higher compared to healthy subjects. Grater level of NO in dialyzed patients than in healthy subject is associated with lower parameters of lung function tests. This can be explained by progression of inflammation, pulmonary oedema also known as ?uraemic lung?or/and the progression of pulmonary hypertension.
Keywords
Reactive nitrogen species, lung function tests, uremia
Introduction
Treatment of end stage renal diseases (ESRD) patients with any mode of dialysis leads to development of complications in most organs and organ systems. The negative effects of uremia are present in the lungs as well. The ventilatory function disorder in this group of patients is mainly presented as obstructive pulmonary disease [1-3]. Besides this, a number of literature provides data on existence of imbalance of vasoactive molecules in uremic patients, primarily nitric oxide (NO) [4?11].
In addition to its powerful vasodilatative effects, NO express bronchoconstrictive effects as well [12,13]. There is a small number of studies which investigated effects of NO on spirometry parameters in patients with ESRD treated with dialysis. The aim of this study was to show the correlation between NO levels and spirometry parameters in ERSD patients treated with hemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD) compared to healthy subjects.
Material and methods (patients)
Three groups of subjects were included in this study. The first group consisted of 28 ESRD patients treated with HD three times per week at the Institute for nephrology of University hospital in Nis [15 males, 13 females, mean age 55.9 (±16, 2) years]. Duration of each HD procedure was between 180 and 240 minutes (individual approach). Hemodialysis machines were produced by Gambro and Fresenius with controlled ultrafiltartion and with usage of acetate and bicarbonate module. Haemodialysis was performed on the following dialyzers: E4H, F6, F60, F60s. Heparinisation was continuous with 4000-5000 i.u. of heparin per patient. None of the patients had primary pulmonary disease, which was concluded after reviewing medical histories and chest x rays. We did not find any other comorbidities that could induce ventilatory failure. None of these patients had haemodynamic instability during haemodialysis.
Second group included 23 patients [10 males, 13 females, mean age 55.8 (±15,8) years] who were treated with CAPD at the Institute for nephrology of University hospital in Nis. Dialysis solution was changed three times per day and patients were trained to do it by themselves or it was done at the Institute under the supervision of the medical staff. None of the patients in second group had primary pulmonary disease which was concluded by the same approach that was used in the first group.
Besides two groups of patients, we included a third group which consisted of 30 healthy subjects [14 males, 16 females, mean age 51.8 (±15,6) years] to serve as a control group. We measured the levels of NO in the third group and its mean level (+/- SD) served as a referent value. All studied subjects where non smokers.
Spirometry
Spirometry parameters were recorded by portable spirometar (Microlab ? micro medical limited 2003). In the group 1 spirometry was performed before haemodialysis procedure, when the interdialysis weight gain (fluid overload) was the highest while in the group 2 spirometry was performed when abdominal cavity was filled with dialysis fluid, just before emptying. This way, both groups of patients were equalized in fluid balance. At the time of measurement both groups of patients had the highest levels of harmful substances in the blood along with the highest interdialysis weight gain. Spirometry procedure was performed bedside on each patient three times and the best result was used. All studied patients where in sitting position during spirometry measuring. One trained technician led this procedure.
Measurement of NO serum levels
The NO level in whole blood is determined by measuring nitrite and nitrate (NO32- u NO22-) production using clasical colorimetric reaction (Griess). Blood samples for the determination of NO concentration were diluted 1:1 (vol/vol) with 0.9% saline, protein-precipitated using 30% ZnSO4, 0.05 ml per ml of blood and centrifuged at 700 g for 10 minutes and frozen at -20°C. Conversion of NO3 2- into NO2 2- was done with nitrate reductase elementary zinc. NO2 2-concentration in serum was determined by classic colorimetric Griess reaction. Briefly, equal volumes of samples and Griess reagent (sulfanilamide and naphthalene-ethylene diamine dihydrochloride) were mixed at room temperature. After 5 min, the absorbance was measured at 546 nm using spectrophotometer. The concentration of nitrite was determined by a standard curve prepared with sodium nitrite.
Statistical analysis
The results were processed using standard statistical method (Student?s t-test for small dependent samples, ?difference method?) and for small independent samples (modification by Cochran & Cox) shown as +/- SD. We tested significance of the difference in mean values between studied groups with an aim to monitor changes in respiratory function parameters as well as the enzymatic activity. We considered the value of p < 0,05 statistically significant.
Results
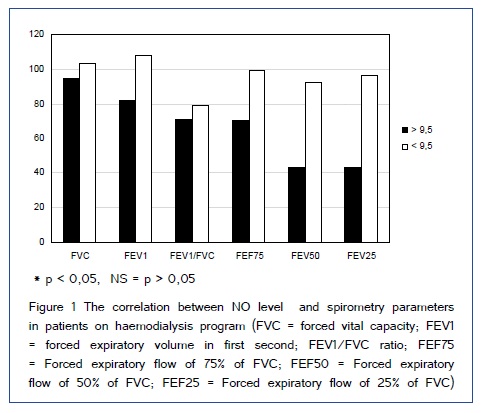
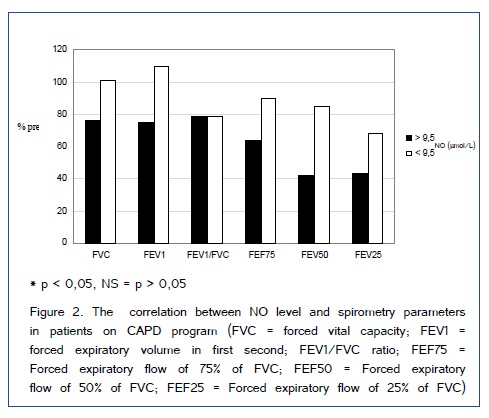
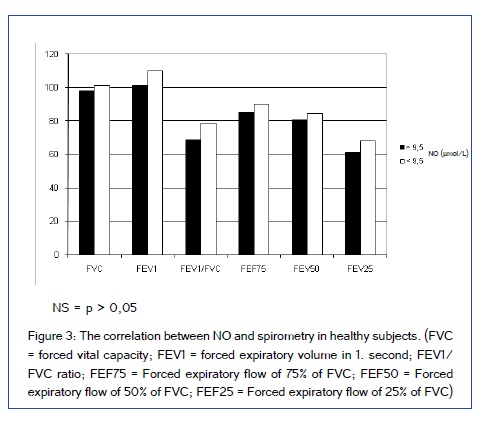
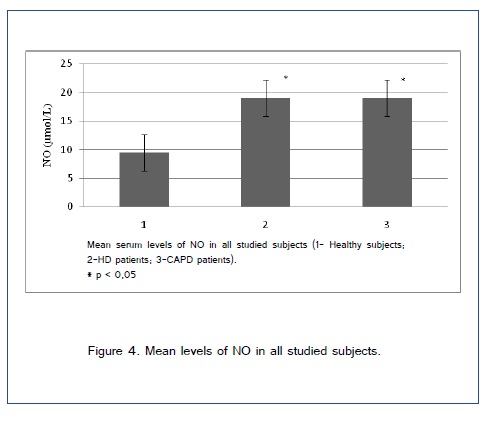
Study included 51 patient on dialysis (HD and CAPD) and 30 patients in control group. Demographic characteristics of included patients are shown in Table 1. Figure [1,2] show comparison of spirometry parameters (in percentages of predicted values) in correlation to NO levels (mean level of NO of 9,5±1,9 ?mol/L in healthy subjects) between patients on HD and patients on CAPD. We found statistically significant difference (p < 0,05) in values of most of pulmonary function parameters in correlation to NO levels in both tested groups of patients. Figure 3 shows comparison of spirometry parameters (in percentages of predicted values) in correlation to NO levels (mean level of NO of 9,5±1,9 ?mol/L) in healthy subjects. There was no statistically significant difference (p > 0,05) in spirometry parameters in control group. Figure 4 presents comparison of mean serum levels of NO in HD group of patients (19,09±6,4), CAPD group of patients (19,09±6,9) and control group (9,5±1,9). Statistical analysis with Student?s t-test for small independent samples (Cochran and Cox modification) showed significantly higher serum levels of NO in HD and CAPD patients in comparison to control group (p < 0,05). Table 2 shows basic and predicted values of parameters of ventilatory function and their correlations in all studied subjects.
| n | male | female | age (years) x(±SD) | Length of dialysis(years)x(±SD) | |
| Haemodialysis. | 28 | 15 | 13 | 55.9 (±16, 2) | 4.14 (±13,3) |
| CAPD | 23 | 10 | 13 | 55.8(±15,8) | 3.4 (±14,7) |
| Control group | 30 | 14 | 16 | 51.8 (±15,6) | - |
Table 1. Basic demographic characteristics of patients
| Ventilatory parameters | Measured basic values | Predicted values | |||
| Haemodialysis patients | |||||
| FVC | 3.967±1.06 | 3.965±0.72 | |||
| FEV1 | 3.025±0.93 | 3.029±0.65 | |||
| FEF25-75 | 3.23±1.73 | 3.27±1.36 | |||
| FEF75 | 6.32±2.13 | 6.97±1.2 | |||
| FEF50 | 3.76±2.05 | 4.86±0.71* | |||
| FEF25 | 1.6±0.85 | 2.05±0.51* | |||
| CAPD patients | |||||
| FVC | 3.36±0.95 | 3.63±0.54 | |||
| FEV1 | 2.6±0.79 | 2.75±0.81 | |||
| FEF25-75 | 2.43±1.02 | 2.91±0.93 | |||
| FEF75 | 4.97±1.57 | 6.4±1.08** | |||
| FEF50 | 2.93±1.44 | 4.54±0.58** | |||
| FEF25 | 1.1±0.53 | 1.9±0.51** | |||
| Healthy subjects | |||||
| FVC | 3.81±0.96 | 3.83±0.83 | |||
| FEV1 | 3.43±0.83 | 2.86±0.68 | |||
| FEF25-75 | 4.75±1.73 | 4.73±1.7 | |||
| FEF75 | 8.35±1.13 | 6.93±0.91 | |||
| FEF50 | 4.5±1.03 | 3.94±0.77 | |||
| FEF25 | 1.96±0.96 | 1.25±0.65 | |||
* p < 0.05, ** p < 0.01
Table 2. Absolute and predicted values of ventilatory function parameters and their correlations in all studied subjects.
Discussion
Spirometry parameters (percentages of predicted values) are significantly lower in patients with NO levels higher than 9,5 ?mol/L compared to patients with NO levels lower than 9,5 ?mol/L in both groups of tested patients. Earlier studies showed that patients treated with regular HD and CAPD had significantly higher levels of NO compared to healthy subjects [37-40]. In our study serum NO values were higher in both dialyzed groups of patients in comparison with healthy subjects. The role of NO in lung diseases is significant and the number of respiratory tract disorders in which pathophysiology of this molecule holds a crucial role is expanding. Besides pulmonary hypertension where NO has considerable effect, asthma, obstructive pulmonary diseases as well as pulmonary fibrosys should be also outlined [17, 35]. In both dialyzed groups of patients we found obstructive and restrictive (reduction of pulmonary volume) lung diseases. Results of other authors who studied spirometry parameters in different dialysis modules are scarce, hence comparison to those is difficult.
Besides all previously listed complications of uremia and its treatment with one of dialysis modules, pulmonary hypertension should also be taken into account. Studies showed that 40% of this population usually develops above mentioned complication. Pulmonary hypertension is accompanied with ventilatory disorders which is reflected in change of spirometry parameters [26]. One of possible reasons for obtained results in this study is the pathophysiological mechanism by which progression of pulmonary hypertension associated with pulmonary fibrosis leads to reduction in spirometry results [36]. The first link in this pathophysiology chain is phenomenon of so called microinflammatory state which is present in this population [27]. The most commonly described causes of this condition were: postsynthetic protein modification [28], oxidative stress [6], type of dialysis membrane or dialysis module , dialysis quality and infection [30,31 ].
It is well known that tumor necrosis factor (TNF-?) is one of leading mediators of inflammation. On the other hand, this inflammation mediator plays significant role in releasing NO from smooth muscle cells of the bronchial tree [32-34]. This cascade of inflammation mediators can affect respiratory function and consequently spirometry parameters. NO have proinflammatory effect, creating a vicious circle of pathophysiologic events. Cumulative effect to bronchial tree can be seen as bronchoconstriction and inflammation accompanied by fibrosis [35]. This fact is supported by studies which showed that patients in terminal stadium of uremia who are treated with regular haemodialysis had NO levels two to six times elevated compared to healthy population [4, 6, 7]. Knowing the effects of endothelin - 1 (ET-1) and NO to pathogenesis of pulmonary hypertension as well as its effects on respiratory function, illustrative are results from few studies which showed that 40% of patients treated with regular haemodialysis have pulmonary hypertension [18?26]. In addition to listed patophysiologic events, endotelin molecule itself along with its physiology can cause these events, because all three forms of ET can cause bronchoconstriction of respiratory tree smooth muscle cells, but ET-1stands out with its bronchoconstrictory effect. The work of group of authors who studied the effects of endothelin in isolated bronchial model showed that ETB receptors placed on smooth muscles of bronchial tree have the highest affinity for ET-1 [14, 15]. These findings are supported by the fact that ETA receptor blockers do not have bronchodilatatory effect, while ETB receptor agonists potentiate bronchoconstrictor effects [12- 16].
It is conceivable to suggest that proinflammatory changes in airway and vascular lung tissue may cause an increase in both ET-1 and NO production and/or release. It is well known that mechanisms leading to an increase in oxidant stress can also lead to the release of ET-1 and production of NO through type II NOS activation. It is also noteworthy that there may be complex interactions between NO and ET-1 in the regulation of vascular tone. Cardillo et al have shown that ETB receptor antagonism decreases the hemodynamic response to NO inhibition in human forearm resistance vessels, indicating that ET-1 is involved in the stimulation of basal NO release through the activation of ETB receptors [41]. Although it is not clear in the present study whether the changes observed in ET-1 levels would influence NO levels, the results, however, implicate that NO and ET-1 may interact to regulate tissue changes during exercise induced bronchospasm.
Some limitations of our investigation should be noted. First, we did not measure diffusing capacity of the lung for carbon monoxide (DLCO) in studied subjects. DLCO is parameter which can contribute to better assessment of pulmonary function. Second, we did not take into account interdialitic weight gain which represent amount of interstitial fluid. Interstitial fluid in the lungs could explain significantly lower values of expiratory flows and the reduction of FVC in dialyzed patients.
From this study it can be concluded that levels of NO can be a marker of clinical deterioration rather than the cause of airway obstruction in patients who are in terminal stage of renal insufficiency and who are treated with one of dialysis methods.
References
11. Stjerniguist M ( 1998) Endothelins?vasoactive peptides and growth factors. Cell Tissue 292: 1?9.
17. Barnes JP, Belvisi GM (1993) Nitric oxide and lung disease.Thorax 48: 1034-1043.
20. Pastan S, Bailey J ( 1998) Dialysis therapy. N Engl J Med 338: 1428-1436.
21. Ifudu O. (1998) Care of patients undergoing hemodialysis. N Engl J Med 339: 1054-1062.