Research Article
Prathibha Shetty1*, Swapnita Pradhan1, and Chandra Viswanathan1
1Regenerative Medicine Group, Reliance Life Sciences Pvt. Ltd., R-282, TTC Area of MIDC, Thane-Belapur Road, Rabale, Navi Mumbai – 400 701, India
Corresponding author
Dr. Prathibha Shetty, MSc, PhD, Regenerative Medicine Group, Reliance Life Sciences Pvt. Ltd., R-282, TTC Area of MIDC, Thane-Belapur Road, Rabale, Navi Mumbai 400 701, India, Tel: 091 2240678352; Fax: 091 2240678099; E-mail: prathibha.shetty@relbio.com
Received Date: 24th January 2015
Accepted Date: 13th February 2015
Published Date: 17th February 2015
Citation
Shetty P, Pradhan S, Viswanathan C (2015) Stem Cell Strategy for the Treatment of Motor Neuron Diseases. Enliven: J Stem Cells Regen Med 2(1): 002.
Copyright
@ 2015 Dr. Prathibha Shetty. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Motor Neuron disease is a neurodegenerative disease of central nervous system leading to death of motor neurons from CNS and spinal cord. Due to the lack of effective treatment modalities, recently, many scientists have considered stem cells as a potential strategy for the treatment of this disease. Amongst the different types of stem cells, MSCs have been thought as a promising source of stem cells in regenerative medicine applications. These cells can be transplanted into the injured nervous system as a new therapeutic strategy. Although many challenges and concerns still remain, and the exact mechanism by which stem cells act is still unknown, the positive effect of stem cells following transplantation has encouraged many researchers to consider this as a treatment option in the future. There has been a lot of research ongoing on the derivation of motor neurons from stem cells in vitro and many scientists are attempting to bring these cells into clinical applications by conducting a number of clinical trials. Here, in this review, we discuss the current progress in pre-clinical studies and the status of clinical trials using stem cells in motor neuron diseases. We also discuss the hurdles which need to be overcome while moving from bench to bedside. Based on current information available, stem cell derived motor neurons can pave the way as a new treatment option for patients with the disease which seems to be highly promising.
Keywords
Mesenchymal stem cells; Neurodegenerative diseases; Motor neuron disorders; Preclinical studies; Clinical trials; Regenerative medicine; Cell therapy
Abbreviations
UCMSCs: Umbilical Cord Mesenchymal Stem Cells; MNs: Motor Neurons; hESCs: human Embryonic Stem Cells; iPSCs: induced Pluripotent Stem Cells; MND: Motor Neuron Diseases
Introduction
Neurodegenerative diseases are an umbrella term used for debilitating conditions that result in progressive degeneration of specific kind of nerve cells. The treatment of various neurodegenerative diseases, such as Alzheimer disease (AD), Parkinson disease (PD), Motor Neuron diseases (MND), and Multiple sclerosis (MS), has become a growing interest in the medical community due to its challenges. Each of these diseases affects different areas and various cells of the central nervous system (CNS) and the spinal cord. These are unmet medical needs which are a great cause of concern for the clinicians.
Motor neuron diseases are a group of fatal neurodegenerative diseases characterized by loss of motor neurons. Unfortunately, MND are diagnosed very late in the course of disease progression. Leading to progressive muscle atrophy and death. In almost all cases, within 3-5 years of diagnosis, the patient suffering from MND dies [1]. The incidence of MND each year is about two people in every 100,000. The prevalence or number of people living with MND at any one time is approximately seven in every 100,000 [2]. Amongst all the MND, Amyotrophic Lateral Sclerosis (ALS) is the most common MND. The current treatments are usually palliative to slow down the disease progression and alleviate the symptoms. Therefore, transplantation of stem cells or their derivatives are being explored as a possible therapeutic option for such patients. In this review, we bring to you a comprehensive report on the status of research on stem cells as a treatment for various types of MND.
Upper motor neurons (UMN) are the motor neurons of brain and brain stem which carry motor information to the lower motor neurons. Lower motor neurons (LMN) are motor neurons that extend from the spinal cord to the muscles. The cell body of a lower motor neuron is located in the spinal cord and it terminates in the skeletal muscle. Each MND has different disease manifestation, different symptoms and a different outcome based on the motor neurons which are affected. MND can be classified into various types such as ALS, the commonest MND, also known as Lou Gehrig’s disease, is characterized by selective, premature degeneration and death of motor neurons in the brain (UMN) and spinal cord (LMN) initiating in mid-adult life. The ensuing progressive paralysis leads to death within a few years as a result of respiratory failure. Amongst all the MND, ALS has incidence rate of 90%. Although the majority of incidences have no apparent hereditary aspect, ~10% of instances are dominantly inherited [3]. ALS patients typically have both LMN and UMN involvement. Worldwide, the incidence rate of ALS varies from approximately 0.3–2.5 cases per year per 100,000 persons [4]. Progressive bulbar palsy, also called progressive bulbar atrophy, involves the brain stem—the bulb-shaped region comprising LMN that are required for swallowing, speaking, chewing, and other functions. Primary lateral sclerosis (PLS) affects the UMN of the arms, legs, and face. It occurs when specific nerve cells in the motor regions of the cerebral cortex (which is the thin layer of cells covering the brain responsible for most high-level brain functions) gradually degenerate. Progressive muscular atrophy (PMA) is marked by slow but progressive degeneration of only the LMN. Spinal muscular atrophy (SMA) is a hereditary disease affecting the LMN. It is an autosomal recessive disorder caused by the defect in the gene SMN1, which makes a protein that is important for the survival of motor neurons (SMN protein) [5] (Table 1).
| Disease name | Affected neurons | Major clinical features | Genes involved | |
| 1 | Amyotropic Lateral Sclerosis (ALS) | motor neurons in the brain (UMN) and spinal cord (LMN) | progressive paralysis, respiratory failure | SOD1, C9ORF72, ALS2, SETX, VAPB, ANG |
| 2 | Progressive Bulbar Palsy (PBP) | Lower motor neurons in the brain stem | pharyngeal muscle weakness, progressive loss of speech, and tongue muscle atrophy | No genes involved |
| 3 | Primary Lateral Sclerosis (PLS) | affects the upper motor neurons of the arms, legs, and face | Movements become slow, pseudobulbar effect | No genes involved |
| 4 | Progressive Muscular Atrophy (PMA) | progressive degeneration of only the lower motor neurons | muscle wasting, clumsy hand movements, fasciculations, and muscle cramps | No genes involved |
| 5 | Spinal Muscular Atrophy (SMA) | hereditary disease affecting the lower motor neurons | weakness and wasting of the skeletal muscles | SMN1, SMN2 |
Table 1: Table showing the pathophysiology and the genes involved in MND
The exact cause of MND is still unknown. But 10% of cases seem to be hereditary. Familial forms of ALS are associated with missense mutations in the gene encoding Cu/Zn superoxide dismutase (SOD1) [7]. A major cytoplasmic antioxidant, the ubiquitously expressed SOD1’s normal function is to catalytically convert highly reactive superoxide (oxygen with an extra electron) to either hydrogen peroxide or oxygen [3]. Other environmental factors such as oxidative stress, glutamate poisoning [1], and factors like aberrant protein aggregation, protein misfolding, neuroinflammation, mitochondrial dysfunction and excitotoxicity are also factors believed to be involved in this disease
(http://www.med.upenn.edu/cndr/AmyotrophicLateralSclerosisALS.shtml).
Also a recently discovered hexanucleotide ‘‘GGGGCC’’ repeat’’ expansion in the noncoding region of the C9ORF72 gene is the most common genetic abnormality and has been found in at least 8% of sporadic ALS (sALS) and frontotemporal dementia (FTD) cases and more than 40% of familial ALS (FALS) and FTD cases. RNA toxicity plays a key role in regulation of C9ORF72 in ALS patients [8].
A cellular model of C9-ALS (familial form) with motor neurons differentiated from induced pluripotent stem cells (iPSCs) derived from ALS patients carrying the C9ORF72 repeat expansion was createdin the study by Sareen D, et al. [9]. Antisense oligonucleotides (ASOs) targeting the C9ORF72 transcript suppressed RNA foci formation and reversed gene expression alterations in C9-ALS motor neurons. This data show that patient derived motor neurons may also be used to delineate pathogenic events in ALS [9]. The identification of the mutations in these genes for each patient by next generation sequencing and the generation of autologous stem cells for correcting the mutation by gene edition technologies are becoming the mainstream and popular advances in personalized medicine today.
The diagnostic tests for MND include blood tests in which the blood sample of the suspected MND patient is analyzed for any rise in a creatine kinase. The naturally-occurring nerve impulses within certain muscles are recorded in Electromyography (EMG), Nerve Conduction Tests, (NCT) and Transcranial Magnetic Stimulation (TMS) which may be carried out at the same time [2].
The only drug licensed by US FDA for the treatment of MND is riluzole, which was designed as a specific glutamate antagonist. It reduces the sensitivity of motor neurons to the neurotransmitter glutamate. Supportive counseling is as important as drug therapy [10]. But till date, there is no effective treatment available for patients suffering from MND. It is important to monitor the quality of life and not just duration of survival, as a part of the assessment of effectiveness of drug regimens. Since other treatment options are just the therapies to slow down disease progression or alleviate symptoms, cell based therapies are required to be explored as a treatment option for MND.
Role of Stem Cells in Neurological Diseases
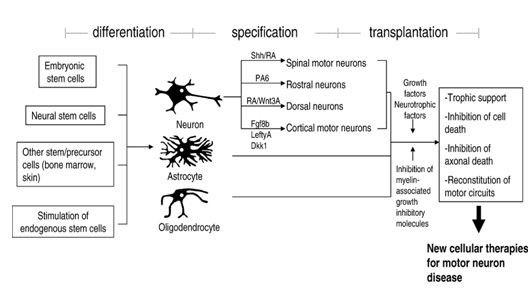
Stem cells derived and expanded from different sources such as adult, embryonic etc. are useful for various therapeutic applications. These exogenously derived cells when transplanted may serve as a therapeutic value in a number of ways such as; they may provide trophic support to host cells, slow a degenerative process, facilitate axonal growth or glial function, secrete neurotransmitters which are deficient in the host, differentiate into oligodendrocytes and myelinate host axons, or differentiate into neurons and form neuronal connections across disconnected populations or replace damaged neuronal circuits [11]. The potential uses of the exogenously and endogenously derived stem cells in neurological diseases may be viewed from different angles. Firstly, the ex vivo derived stem cells can be utilized as a biological tool to understand the pathway of various neurological disorders. Secondly, the potential of endogenous stem cells present in the mammalian nervous system can be harnessed and expanded to explore the mechanisms involved in the repair of the damaged tissue. By identifying the cues that guide the endogenous stem cell functions, researchers shall be able to modulate their function and mimic them in an in vitro condition. This will lead to elucidate the functionally-relevant neural tissue repair in MND. By understanding these mechanisms, stem cells or committed progenitors derived from stem cells may be useful in transplantation into the injured nervous system as a therapeutic strategy in future.
Research with ESC and iPSC Derived Motor Neurons
Several groups have differentiated human or mouse embryonic stem cells to motor neurons with high efficiency. Takazawa et al. and Li et al. [12,13] have derived mature spinal motor neurons from hESC. The in vivo studies by Wichterle et. al. showed, in preclinical model that ES cell-derived MNs are able to negotiate successive steps in the normal developmental program through which a newly generated MN in the spinal cord innervates its skeletal muscle target [14].
For the first time in 2009, Karumbayaram et al. [15] demonstrated that human iPSC derived cells are able to generate electrically active motor neurons in vitro. Su H et. al [16] in 2013 demonstrated that human iPSCs derived from mesenchymal cells of the umbilical cord possessed a higher capability in differentiation to MNs. These cells when transplanted into the injured musculocutaneous nerve of rats, they survived robustly, extended axons along the nerve, and formed functional connections with the target muscle, thereby protecting the muscle from atrophy. These studies provides evidence for the first time that human iPSC-derived motor neurons are truly functional not only in vitro but also in vivo, and they have the potential for stem cell-based therapies for MND [16].
However, reprogramming methods need improvement to derive clinically safe and highly effiecient iPSCs. iPSC and hESC derived motor neurons can be used in vitro for disease modeling and drug discovery and screening. Pluripotent cells such as hESCs and iPSCs have the ability to differentiate into cells of all three germ layers, but due to the controversies and ethical issues, both these cell types have limitations in clinical applications.
Adult Stem Cells in Motor Neuron Diseases and their Possible Role in Treatment
Mesenchymal Stem Cells (MSCs) are non-hematopoietic multipotent stem cells that are capable of differentiating into both mesenchymal and non-mesenchymal lineages. In fact, in addition to bone, cartilage, fat, and myoblasts, it has been demonstrated that MSCs are capable of differentiating into neurons and astrocytes in vitro and in vivo [17]. Interestingly, MSCs also have been differentiated to motor neurons by various researchers. The properties of MSCs can be explored for successful autologous as well as allogenic transplantations. Several clinical trials with MSC for ALS have been reported (Table 3). A myriad of methods of transplantation have been investigated in the past and are being investigated now in animal models and clinical trials as potential therapeutic candidates for the treatment of MND. Although many challenges and concerns in preclinical experiments and early human trials still remain, the beneficial effects observed so far following transplantation have sparked a new hope for MND patients [6], MSCs possibly secrete a variety of factors that influence neurological healing, neuroprotection, and regeneration [18]. Upon transplantation into the brain, MSCs are believed to promote endogenous neuronal growth, decrease apoptosis, reduce levels of free radicals, encourage synaptic connection from damaged neurons and regulate inflammation, primarily through paracrine actions. They also to promote functional recovery by producing trophic factors that induce survival and regeneration of host neurons [19]. Sun et al. [16] demonstrated that MSCs modulate motor neuron and glial response to apoptosis and inflammation. Also the study has shown that the factors secreted by MSC in the conditioned media (CM) exert their effects on all the different types of cells of the body. The growth factors and cytokines secreted by these cells are also shown to reverse the symptoms of various types of degenerative diseases such neurological, vascular, muscular etc. The data indicate that MSC CM exerts a protective effect against in vitro induced apoptosis in motor neurons. Thus, these cells are an interesting candidate for preclinical studies in animals and clinical evaluation in ALS patients [20] (Figure 1 and Figure 2).
| Source of cells | Site of injection | No. of clinical trials/phase | Recruitment status/ number of patients | Country | Current Primary outcome measure/ study reports |
| Autologous Bone marrow derived stem cells | intra venous injection by stereotaxis | Phase I | Completed; 6 patients enrolled | Tehran, Iran | Safety evaluation |
| intraventricular injection of by stereotaxis | Phase I | Not yet recruiting; 10 patients | Tehran, Iran | Safety evaluation | |
| intrathecal | Phase I | Recruiting; 10 patients | Tehran, Iran | Safety evaluation | |
| lumbar puncture into cerebrospinal fluid | Phase I | Recruiting; 25 patients | Minesota, United States | Number of patients with dose-limiting toxicities | |
| Intrathecal (IT) transplantation | Phase I and II | Completed; 71 patients | Seoul, Korea | comparison of efficacy and safety between test group and control group | |
| Intrathecal injection | Phase I and II | Not yet recruiting; 5 patients | Isfahan, Iran | Efficacy of the transplant | |
| Intrathecal injection | Phase II | Enrolling by invitation; 50 patients | Szczecin, Poland | Safety by repeated follow-up over one year with clinical and laboratory evaluations | |
| one-time intrathecal infusion of the cell product suspended in infusion medium | Phase I | Suspended; 6 patients | United States | Safety as assessed by absence of complications at the site of infusion or the appearance of new neurologic deficit not attributed to the natural progression of the disease. | |
| intraspinal and intrathecal infusion | Phase I and II | Active, not recruiting; 63 patients | Murcia, Spain | No acceleration in decline noted; increased motor neuron numbers noted in treated spinal cord segments at autopsy; motor neurons surrounded by CD90+ cells without degenerative ubiquitin deposits | |
| multiple intramuscular injections at 24 separate sites, in addition to a single intrathechal injection into the CSF | Phase II | Active, not recruiting; 14 patients | Jerusalem, Israel | Safety evaluation and tolerability of a single treatment administration in an escalating-dose | |
| Intramuscular and intrathecal injection | Phase II | Completed; 12 patients | Jerusalem, Israel | Changes in the progression rate of the disease as evidenced by changes in the ALS functional rating scale (ALS-FRS) | |
| Intraspinal | Study completed | Completed; 9 patients | Italy | High thoracic (T7-T9) injections; no apparent toxicity, transplant related adverse events, | |
| or structural changes; evidence of slowed functional decline in 4 patients; follow-up of 4and up to 9 years | |||||
| Intraspinal | Study completed | Completed; 10 patients | Italy | High thoracic (T4-T6) injections; no apparent toxicity, transplant related adverse events or structural changes; follow-up of 2 to 5 years | |
| Umbilical Cord Mesenchymal Stem Cells | lumbar puncture | Phase II | Study start: March 2012; Estimated Study Completion : April 2015 | Beijing, China | Nerve function evaluation |
| Autologous BM-hematopoetic stem cells | Intrathecal | Phase II, III | Active, not recruiting; 71 patients (Feb 2011-Aug 2013) | Mexico | Evaluation of intrathecal administration |
| Autologous adipose tissue derived MSC | lumbar puncture into the cerebrospinal fluid | Study completed | Completed; 1 patient (June 2010-April 2011) | United states | Clinical monitoring of possible reaction |
| Human neural stem cells | spinal cord | Phase I | Recruiting; 18 patients (Dec 2011-Spet 2016) | Terni, Italy | to verify safety and tolerability |
| Human Spinal Stem Cells (HSSC) | spinal cord after laminectomy | Phase I | Active, not recruiting; 18 patients (Jan 2009- Dec 2015) | United States | to determine the safety |
| iPSCs | None | ||||
Figure 1: Differentiation of stem cell into neuronal type and applications of cellular therapies in motor neuron disease [11]
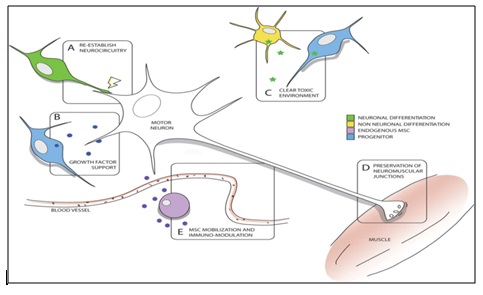
Figure 2: Potential mechanisms of stem cell efficacy in motor neuron diseases. Within the spinal cord, stem cells that differentiate into neurons can synapse with existing motor neurons to re-establish or maintain neurocircuitry (A) and provide neurotrophic support (B). Differentiations of stem cells into non-neuronal cell types can also impact attenuating oligodendrocyte dysfunction and mitigating toxicity (C). In the periphery, stem cell transplantation into muscle can provide support to maintain functional neuromuscular junctions (D). And the mobilization of endogenous MSCs from the bone marrow into the circulation can also induce immunomodulatory effects that attenuate inflammatory responses within the spinal cord (E) [21].
Preclinical Studies with Stem Cells in Motor Neuron Diseases
Several groups have transplanted stem cells in transgenic MND animal model and have reported various outcomes. So far, it has been demonstrated that stem cell therapies show motor neuron differentiation in transgenic ALS spinal cord [1]. Also, various scientists have shown in animal models and clinical trials of ALS that stem cells significantly slow the progression of the disease and prolong survival. In majority of the preclinical studies, mutant animal models with defective gene SOD1gene were used for studying the effective transplanted cells.
The following table describes a partial list of studies using stem cells derived transplants in various animal models [1,7,20] (Table 2).
| Animal Model | Cell Type Grafted | Route of administration | Effect on Disease Progression | Reference |
| SOD1G93A mouse | hMSC | intravenous | Extended lifespan, delayed disease onset, prolonged rotarod performance, protected endogenous MNs, preserved sciatic nerve compound muscle action potential (CMAP) amplitude | 22 |
| hMSCs or hMSC-derivedneural stem-like cells(hMSC-NSCs) | intrathecal into cisternamagna | No effect on: lifespan, disease onset, endogenous MN survival and performance on running wheel | 23 | |
| hMSCs | intraspinal | Delayed motor score decline, protected endogenous MNs (35%), decreased microgliosis,decreased astrogliosis, prolonged rotarod performance | 24 | |
| GFP tagged hMSCs hMSCGFP | 3 injections at 1week intervals, intramuscular | Extended lifespan (~28 days), no effect on disease onset, maintained large endogenous motor neurons, delaydenervation from neuromuscular junction, delayed decline in Blood Brain Barrier | 25 | |
| hUCBCs(human Umbilical Cord Blood Cells) | retro-ocular, intravenous | Extended lifespan, decreased levels of proinflammatory cytokines in the lumbar SC, decreased microglial cell density in cervical and lumbar SC | 26 | |
| hMSC | intrathecal into cisternamagna | increase in lifespan and MN survival | 27 | |
| Musculocutaneous nerve lesion in rat | iPSC derived motor neurons | injured musculocutaneous nerve of rats | Motor neurons survived robustly, and formed functionalconnections with the target muscle (biceps brachii), thus protecting the muscle from atrophy | 16 |
In the preclinical studies, the cells have been administered through various routes such as intrathecal, intravenous, intraspinal, intramuscular, retro-ocular and retro-orbital. Various studies have been carried out using MSCs and iPSCs via all possible routes and these cells were found to be safe and efficacious and after multiple injections, there were no side-effects observed. The published results have also shown that there was extended lifespan, delayed disease onset and increase in motor neuron survival in the animals post stem cell transplant. (The sentence has been modified)
Current Clinical Trials on Motor Neuron Diseases
On the basis of the pre-clinical studies, Mazzini et al. [23] in 2003 conducted a study to verify the efficacy of MSC transplantation in patients with ALS. This research group enrolled seven patients with ALS, showing severe functional impairment of lower limbs and mild impairment of upper limbs. Three months after cell implantation, a mild trend toward a slowing down of decline of muscular strength was observed in the proximal muscle group of lower limbs in four patients. Again, in 2008, Mazzini et al. [30] published data obtained from the trial in which MSCs were autologously transplanted to ALS patients through spinal cord injection. However, this study was carried out only on nine patients in order to confirm the previous results. In 2010, this group replicated the study with ten patients. Their objectives were to assess the feasibility and toxicity of MSC transplantation and to test the impact of a cell therapy in ALS patients. This time too, similar results were obtained and there were not enough changes in the progression of the disease as compared to their own previous experiments. A trial in 2012 by the same group on 19 patients with ALS demonstrated that direct injection of MSCs into the thoracic spinal cord was safe. And this study is the first to show the safety of MSC transplantation in the central nervous system after a long time monitoring of nearly 9 years, and is in support of using MSC-based cellular therapy for neurodegenerative disorders [28-32] (Table 3).
Challenges
Before heading towards clinical application of stem cells, there are many hurdles to overcome. A lot of research needs to be done to understand whether the terminally differentiated or the progenitors will be the ideal cells for treatment of the disease. Also study needs to be done to understand the probable mechanisms by which transplantation of these cells will lead to enhanced functional recovery. In addition, the appropriate type and amount of stem cells as well as the segmental locations and number of graft sites will need to be optimized for attaining the most efficacious transplants. One can easily compute the number of studies that will be needed to arrive at some reasonable conclusion. Intrathecal implantation may permit cells to circulate via the CSF, expecting efficient generation of neurons, particularly motor neurons [1]. While there has been great interest in the stem cell technology that has been applied in a number of clinical trials, the pathological environments of neurodegenerative diseases needs to be assessed better alongside the evidence of cells staying there for variable durations. This will throw some light on how neuronal circuitry can be maintained by the transplant cells [22].
Regulatory Concerns in the Area of Stem Cell Research and Therapy
>
The regenerative medicine industry faces amongst other challenges huge funding, leading scientists to rely on industry funding for the translation of research and clinical trials especially in such areas. Translating basic stem cell research into preclinical studies and then to clinical trials are a complex multi-step process which entails the challenge related to the results obtained and complying with the current regulations and guidelines.
The growing global interest in stem cell research & therapy prompts development of a sturdy regulation and oversight along with steps to enhance public knowledge and awareness. In India, there are multiple agencies which directly or indirectly govern stem cell science i.e. Department of biotechnology (DBT), Indian Council of Medical Research (ICMR) and the Drug Controller of India (DCGI). For Stem Cell Research and Therapy, Indian Council of Medical Research and Department of Biotechnology had released guidelines in 2007. Considering the developments in the field, the 2007 guidelines have been revised and finalized in December 2013 and named as National Guidelines for Stem Cell Research. The efforts of the ISSCR (International Society for Stem Cell Research) stem cell standards committee in facilitating international guidelines and standards is a reflection of the global realization and harmonization in the area of regenerative medicine. There should be adequate steps to address challenging issues such as these, with a specific intension of fast tracking the developments; as each milestone could take very long periods. On humanitarian basis, clinical trials even on fewer cases can offer valuable learning that will certainly be contributory to the development of stem cell based therapies. Due to these regulatory uncertainties, the fraternity of scientists, translational researchers and clinicians will have to wait for several years before stem cells becomes a prescription for treating various degenerative disorders.
Proposed Strategy for Treatment of MND using Stem Cells
Multiple cell type delivery has the potential to provide support through many different mechanisms. Engrafting a not fully differentiated or progenitor stem cell population can positively impact the outcome of the study along with the safety of the transplant. There could be a multi potential approach which may slow down the progression of the disease which may provide a critical support to the neurons in the brain. The stem cells which are administered could be implanted into the patient and accepted among the native body cells which could be functionally active. For this, cells could be administered using more than one routes and more than a dose. Hence a combinatorial strategy using different cell types can be followed which will lead to long term survival and engraftment of stem cells. These approaches could be explored for a successful stem cell treatment modality in future.
Future
Regenerative medicine will change the nature of treatment for various unmet medical needs in the future. It will demand highly specialized hospitals or day centers, skilled technologists and cell therapists to perform cell implantation using autologous and allogeneic cells. Health care systems will need much more thought of costs, conductive regulations, insurance policies etc. By inviting private sectors to represent themselves in various governments level meetings, and contribute to the cutting edge of technology, will help ensure that they are workable and effective. Such developments alone can help nurturing of regenerative medicine to become the third arm of pharmaceuticals alongside small molecule drugs and biopharmaceuticals.
Acknowledgements
The authors acknowledge Reliance Life Sciences Pvt. Ltd (www.rellife.com), for providing the infrastructure and financial support to work on this project.
References
- Thonhoff JR, Ojeda L, Wu P (2009) Stem Cell-Derived Motor Neurons: Applications and Challenges in Amyotrophic Lateral Sclerosis. Curr Stem Cell Res Ther 4: 178-199.
- www.mndassociation.org/
- Ilieva H, Polymenidou M, Cleveland DW (2009) Non–cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol 187: 761-772.
- Pratt A, Getzoff E, Perry JJ (2012) Amyotrophic lateral sclerosis: update and new developments. Degener Neurol Neuromuscul Dis 2: 1-14.
- www.ninds.nih.gov/
- Van Den Bosch L, Timmerman V (2006) Genetics of Motor Neuron Disease. Curr Neurol Neurosci Rep 6: 423-431.
- Gowing G, Svendsen C (2011) Stem Cell Transplantation for Motor Neuron Disease: Current Approaches and Future Perspectives. Neurotherapeutics 8: 591-606.
- Donnelly CJ, Zhang PW, Pham JT, Heusler AR, Rothstein JD, et al. (2013) RNA Toxicity from the ALS/FTD C9ORF72 Expansion Is Mitigated by Antisense Intervention. Neuron 80: 415-428.
- Sareen D, O'Rourke JG, Meera P, Muhammad AK, Grant S, et al. (2013) Targeting RNA foci in iPSC-derived motor neurons from ALS patients with C9ORF72repeat expansion. Sci Transl Med 5: 208ra149.
- Talbot K (2002) Motor neuron disease. Postgrad Med J 78: 513-519.
- Nayak M, Kim Y, Goldman M, Keirstead H, Kerr DA (2006) Cellular therapies in motor neuron diseases. Biochim Biophys Acta 1762: 1128-1138.
- Takazawa T, Croft GF, Amoroso MW, Studer L, Wichterle H, et al. (2012) Maturation of Spinal Motor Neurons Derived from Human Embryonic Stem Cells. PLoS One 7: e40154.
- Li WW, Wei YH, Li H, Lai DM, Lin TN (2013) Isolation and Characterization of a Novel Strain of Mesenchymal Stem Cells from Mouse Umbilical Cord: Potential Application in Cell-Based Therapy. PLoS ONE 8: e74478.
- Wichterle H, Lieberam I, Porter J, Jessell T (2002) Directed Differentiation of Embryonic Stem Cells into Motor Neurons. Cell 110: 385-397.
- Karumbayaram S, Novitch B, Lowry W (2009) Directed Differentiation of Human-Induced Pluripotent Stem Cells Generates Active Motor Neurons. Stem Cells 27: 806-811.
- Su H, Wu W (2013) Transplanted motoneurons derived from human induced pluripotent stem cells form functional connections with target muscle. Stem Cell Research 11: 529-539.
- Pittenger M, Mackay A, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage Potential of Adult Mesenchymal Stem Cells. Science 284: 143-147.
- Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG (2006) Human mesenchymal stem cell population express a variety of neuro regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol 198: 54-64.
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, et. al. (2010) Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 5: 933-946.
- Sun H, Be´nardais K, Stanslowsky N, Thau-Habermann N, Hensel N, et al. (2013) Therapeutic Potential of Mesenchymal Stromal Cells and MSC Conditioned Medium in Amyotrophic Lateral Sclerosis (ALS) - In Vitro Evidence from Primary Motor Neuron Cultures, NSC-34 Cells. Astrocytes and Microglia. PLoS One 8: e72926.
- Lunn JS, Sakowski SA, Hur J, Feldman EL (2011) Stem Cell Technology for Neurodegenerative Diseases. Ann Neurol 70: 353-361.
- Xiao Q, Zhao W, Beers DR, Yen AA, Xie W, et al. (2007) Mutant SOD1G93A microglia are more neurotoxic relative to wild-type microglia. J Neurochem 102: 2008-2019.
- Habisch HJ, Janowski M, Binder D, Kuzma-Kozakiewicz M, Widmann A, et al. (2007) Intrathecal application of neuroectodermally converted stem cells into a mouse model of ALS: limited intraparenchymal migration and survival narrows therapeutic effects. J Neural Transm 114: 1395-1406.
- Vercelli A, Mereuta O, Garbossa D, Muraca G, Fagioli F, et al. (2008) Human mesenchymal stem cell transplantation extends survival, improves motor performance and decreases neuroinflammation in mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 31: 395-405.
- Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, et al. (2007) GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One 2: e689.
- Chen R, Ende N (2000) The potential for the use of mononuclear cells from human umbilical cord blood in the treatment of amyotrophic lateral sclerosis in SOD1 mice. J Med 31: 21-30.
- Kim H, Kim HY, Choi MR, Hwang S, Nam KH, et al. (2010) Dose-dependent efficacy of ALS-human mesenchymal stem cells transplantation into cisterna magna in SOD1-G93A ALS mice. Neurosci Lett 468: 190-194.
- Mazzini L, Fagioli F, Boccaletti R, Mareschi K, Olivieri C, et al. (2003) Stem cell therapy in amyotrophic lateral sclerosis: a methodological approach in humans. Amyotrophic Lateral Scler Other Mot Neuron Disord 4: 158-161.
- Mazzini L, Mareschi K, Ferrero I, Vassallo E, Oliveri G, et. al. (2006) Autologous mesenchymal stem cells: clinical applications in amyotrophic lateral sclerosis. Neurol Res 28: 523-526.
- Mazzini L, Mareschi K, Ferrero I, Vassallo E, Nasuelli N, et al. (2008) Stem cell treatment in amyotrophic lateral sclerosis. J Neurol Sci 265: 78-83.
- Mazzini L, Vercelli A, Mareschi K, Ferrero I, Testa L, et al. (2009) Mesenchymal stem cells for ALS patients. Amyotroph Lateral Scler 10: 123-124.
- Mazzini L, Ferrero I, Luparello V, Rustichelli D, Gunetti M, et al. (2009) Mesenchymal stem cell transplantation in amyotrophic lateral sclerosis: A Phase I clinical trial. Exp Neurol 223: 229-237.
- Mazzini L, Mareschi K, Ferrero I, Miglioretti M, Stecco A, et al. (2012) Mesenchymal stromal cell transplantation in amyotrophic lateral sclerosis: a long-term safety study. Cytotherapy 14: 56-60.
- Rossi SL, Nistor G, Wyatt T, Yin HZ, Poole AJ, et al. (2010) Histological and Functional Benefit Following Transplantation of Motor Neuron Progenitors to the Injured Rat Spinal Cord. PLoS One 5: e11852.
- www.alsa.org/