Research Article
Authors:
Luke T. Daum1*, Richard F. Schuman2, Clara J. Sei3, Nimisha Rikhi3, Afia Mesadieu3, John D. Rodriguez1, James P. Chambers1, and Gerald W. Fischer3
1 Longhorn Vaccines and Diagnostics, San Antonio, Texas, USA
2 Antibody and Immunoassay Consultants, Rockville, Maryland, USA
3 Longhorn Vaccines and Diagnostics, Gaithersburg, Maryland, USA
Corresponding author
Luke Thomas Daum Ph.D., Longhorn Vaccines and Diagnostics, San Antonio, Texas, USA, Tel: +1.210.392.6244; E-mail: longhorn-vandd@sbcglobal.net
Received Date: 23 September 2017; Accepted Date: 28 October 2017; Published Date: 05 November 2017
Citation
Daum LT, Schuman RF, CJ Sei , Rikhi N, Afia M, Rodriguez JD, Chambers JP, and Fischer GW Fischer GW (2017) Rapid qPCR Detection of Mycobacterium Tuberculosis in Blood and Organ Tissues Using a Collection-to-Detection System. Enliven: Microbes and Microbial Techniques 4(1):002.
Copyright
@ 2017 Luke Thomas Daum This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Mycobacterium tuberculosis (MTB) is an important cause of sepsis in individuals with HIV and they often die within 18 days of presentation. Thus, there is an urgent need to rapidly detect the presence of MTB bacteremia.
Objective: To demonstrate the potential usefulness of a ‘collection-to-detection’ system for rapid qPCR detection of MTB in blood and organ tissue specimens transported in molecular medium to a distant lab.
Methods: A total of 39 blood and tissue specimens were collected from mice (n=6) given intraperitoneally either anti-MTB monoclonal antibodies (MABS) or PBS, and challenged 24 hours later with an intravenous injection of 105 ethanol-killed MTB. Whole blood and tissue specimens were transported to a distant laboratory for qPCR analysis.
Results: MTB was detected by qPCR in both blood and tissues transported over 1000 miles at ambient temperature within 6 hours of arrival. Differential MTB clearance was observed using two anti-MTB opsonic MABs.
Conclusion: This study supports the usefulness of transporting blood and tissue to a centralized or regional laboratory for expedient diagnosis of MTB bacteremia in high risk patients.
Introduction
The convergence of human immunodeficiency virus (HIV) and tuberculosis (TB) in many areas of the world contributes to increased drug resistance and rapidly progressive disease [1,2]. In sub-Shararan Africa, MTB is now a leading cause of community acquired bloodstream infection among hospitalized patients [3-6]. In HIV patients presenting with severe sepsis, MTB was the most prevalent pathogen detected in blood [5]. Patients with HIV and MTB bacteremia exhibited a 30 day mortality of approximately 50% [3,5], and in one study half of the deaths occurred within 18 days, making blood culturing impractical for detecting MTB bacteremia [5]. In contrast to culturing, real-time quantitative PCR (qPCR) could afford a more rapid diagnosis of MTB from blood. However, in low resource countries many hospitals lack qPCR capabilities. Thus, an easy to use ?on-site? means of collection in combination with safe transport to a regional or centralized laboratory facility capable of qPCR analysis would be very useful in resource limited environments.
A commonly exploited MTB target for sensitive MTB detection is the IS-6110 multi-copy gene (16-20 genomic copies).Previous reports have described PCR and hybridization assays targeting the IS-6110 gene for MTB diagnosis from blood [7,8]. However, strains containing few or no IS-6110 gene copies have been reported [9,10]. Conversely, the IS-1081 geneisless variable in sequence and copy number with most MTB genomes containing approximately sixIS-1081 copies. A Ready-to use, multiplex MTB qPCR assay mixture containing buffers, primers, probe, and enzyme targeting both IS-6110 and IS-1081 has been developed [11]. In this study, using a mouse model we explored the utility of shipping blood and tissues in a molecular transport medium to a distant laboratory for rapid MTB detection using a ready-to-use multiplex qPCR assay mixture.
Materials and Methods
Mouse Bacteremia Model
Ethanol killed MTB (EK-MTB) Erdman strain (ATCC 35801) provided by Battelle Laboratories at 108 CFU/mL was washed three times in phosphate buffered saline (PBS), and diluted to 5x105 CFU/mL. Female BALB/c mice (Harlan Laboratories, Indianapolis, IN) weighing approximately 30 grams (n=6) were given an intravenous injection of 0.2 m LEK-MTB (105 CFU/mouse). Monoclonal antibodies (MABs) that bind MTB as determined by ELISA, demonstrating opsonic activity using HL60 cells (Sei et al., unpublished data) were used to mediate MTB clearance from blood and tissues in order to detect MTB bacilli over a broad range. Two mice in each treatment group were given intra peritoneal injections of either 0.3 mL sterile phosphate buffered saline (PBS), or 0.3mL of PBS containing either 26 µg MAB AB9, or 205 µg MAB GG9 24 hours before MTB challenge. Mice were bled retro-orbitally at 15 minutes post-MTB challenge, and subsequently at 4 and 24 hours by cardiac puncture immediately post-euthanasia. This mouse protocol was approved by the Institutional Animal Care and Use Committee (IACUC protocol 73).
For qPCR, 0.2mL whole blood was transferred to tubes containing EDTA (Becton Dickinson, Sparks, MD), and after all mice were bled 100 µL samples were transferred to 1.0 mL Prime Store Molecular Transport Medium® (PS-MTM). Murine tissues (~1 gram kidney, liver, lung and spleen) obtained at 4 and 24 hours post MTB-challenge were homogenized in PBS, and transferred to tubes containing 1.0 mL PS-MTM.A total of 39 specimens (15 blood and 24 tissue homogenates) were transported over night via Fed Exat ambient temperature from Gaithersburg, MD to San Antonio, TX for qPCR analysis.
DNA Extraction and Multiplex qPCR
Murine blood and tissue homogenates were subjected to nucleic acid extraction using Prime Xtract (Longhorn Vaccines and Diagnostics, San Antonio, TX). For DNA extraction, PS-MTM treated samples(e.g., blood and tissues) were thoroughly mixed for 1 minute by inversion after which time a 0.2 mL aliquot was removed, transferred to 0.2 mL Lysis Solution to which 0.2 mL 100% ethanol was added, and thoroughly mixed for five minutes at room temperature. Following mixing, samples were centrifuged for one minute at ~2,000 xg in order to pellet red blood cells, and the resulting supernatant was decanted, and transferred to spin columns per manufacturer?s instruction. Elution of nucleic acid was achieved by application of 50 µL heated nuclease-free water to the column. Real-time qPCR was carried out in replicates of three for each sample following addition of 2.5?L total DNA(~1-10 ng/µL) to 7.5?L Prime Mix® Multiplex MTB (Longhorn Vaccines and Diagnostics, LLC, San Antonio, TX, USA) and applied to an ABI 7500 Instrument (Thermo Fisher Scientific, Waltham, MA, USA). Results were analyzed using a CT threshold baseline of 0.1 with start cycle = 3, and end cycle = 15. Results were plotted as CT mean values with standard error. The qPCR testing was completed within six hours of specimen arrival.
Results
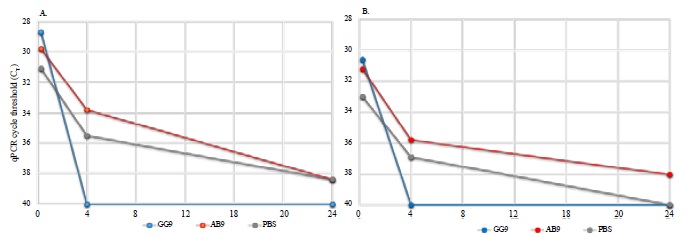
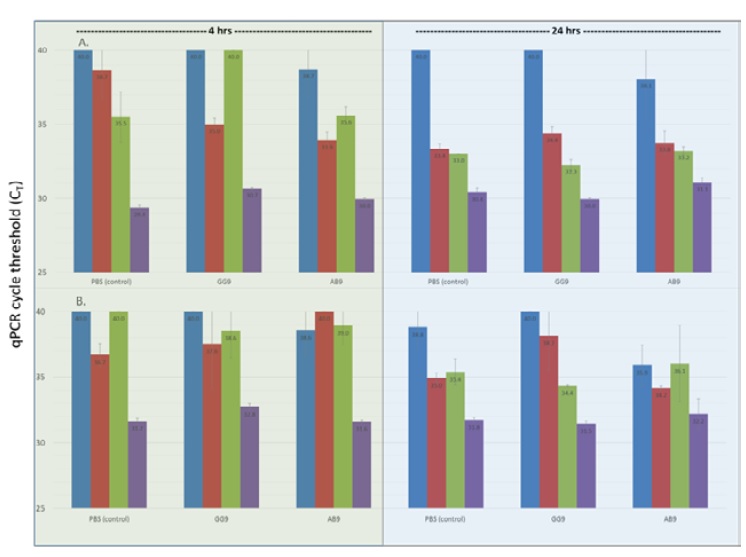
MTB in mouse blood was detected across a broad concentration range according to qPCR (Figure 1). Blood samples obtained 15 minutes post MTB challenge were qPCR positive for MTB with CT mean values of 29.8 (range 28.7-31.1), and 31.6 (range 30.6-33.0) for IS-6110 and IS-1081, respectively (approximately 103 to 104 CFU/mL). As expected, higher IS-6110 copy number provided lower CT values. A rapid decline in bacilli, as indicated by increasing CT values was observed over the first 4 hours with continued reduction in CT values at 24 hours post treatment (Figure 1). At 24 hours, all mice exhibited low levels of MTB in blood, i.e., CT values of 38.9 and 39.5 for IS-6110 and IS-1081, respectively (approximately 101 to 102 CFU/mL). Mice given MAB GG9 cleared MTB from the blood by 4 hours (CT= 40 for IS-6110 and IS-1081 gene targets) at both 4 and 24 hours (Figure 1). PBS treated mice did not clear MTB at either 4 or 24 hours [IS-6110]; however, the less sensitive gene target IS-1081 exhibited a CT value of 40 at 24 hours indicating no detection. MTB was also detected by qPCR in tissue homogenates, i.e., kidney, liver, spleen, and lung with lung tissue exhibiting the highest MTB concentration, i.e., lowest CT values at both 4 and 24 hours post challenge (Figure 2). In contrast, kidney tissue exhibited the highest CT value at 4 and 24 hours indicating few bacilli present at either time. Liver and spleen tissue homogenates exhibited decreasing CT values at 4 and 24 hours is consistent with increased numbers of bacilli, and phagocytes is in these tissues.
Figure 1:Detection of M. tuberculosis using qPCR targeting the IS-6110 (Frame A) and IS-1081 (Frame B) MTB genes. Total DNA was extracted from blood; treatment group MAB GG9 (blue), MAB AB9 (red), and phosphate buffered Saline (PBS) control (gray) before MTB challenge as described in the ?Materials and Methods?.
Figure 2:qPCR detection of M. tuberculosis from murine kidney (blue), liver (red), spleen (green), and lung (purple) DNA targeting IS- 6110 (Frame A) and IS-1081 (Frame B) according to treatment and time after MTB challenge. qPCR was performed for each tissue in duplicate with average mean and standard error bars shown. A qPCR cycle threshold (CT) value of 40 represents no detection.
Discussion
The presence of MTB in the blood was reported over 100 years ago [12]. However, with the convergence of HIV and TB in many parts of the world, MTB sepsis has become more common. Recent studies from Tanzania and Uganda have documented that bacteremic disseminated TB is rapidly fatal, especially with immunologically advanced HIV disease [3,5]. Since MTB is now a common cause of sepsis, rapid and sensitive detection of MTB in blood is urgently needed [5]. Data reported here using a novel mouse model suggest that MTB bacteremia in blood and tissues can be rapidly assessed in transported samples by qPCR. As such, this could be very helpful in: 1) early confirmatory diagnosis, and 2) monitoring therapeutic efficacy. Importantly, while qPCR is a rapid and sensitive diagnostic method not all facilities in low resource countries can perform such molecular testing. This multiplex assay described here uses not only the IS-6110 multi-copy target, but also the highly conservedIS-1081 target. This could be critical for IS-6110 gene deleted strains [9,10]. Furthermore, data from Tanzania suggest that the yield in CFU from blood cultures are lower than the detection limit of Cepheid?s Gen Xpert [13,14]. A recent study documented that Gen Xpert had a 21% sensitivity for diagnosing MTB bacteremia [15]. Furthermore, all individuals that were Gen Xpert positive died, likely indicating high level bacteremia in these patients [15]. It would be desirable to also rapidly identify people with low level bacteremia and begin appropriate treatment before the disease escalates to severe sepsis with a high mortality.
The collection, transport, extraction and qPCR multiplex MTB assay used in this study has been previously validated for sensitive detection of MTB in sputum [16-19]. Here we demonstrate that mouse blood and tissue specimens can be transported at ambient temperature to a distant facility for qPCR analysis, i.e., within one day of specimen arrival.
Although a mouse bacteremia model was used in the study, this approach could significantly impact the diagnosis and treatment of MTB bacteremia and sepsis in patients in many low resource areas where hospitals lack treatment and molecular detection infrastructure. With certainty, clinical studies are needed in high risk patients in Africa using the collection to detection approach described here.
References
- World Health Organization (2015) Global Tuberculosis Report.
- Corbett EL, Marston B, Churchyard GJ, De Cock KM (2006) Tuberculosis in sub-Saharan Africa: Opportunities, challenges and change in the era of antiretroviral treatment. Lancet 367:926-937.
- Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, et al. (2012) Bacteremic Disseminated Tuberculosis in sub-Saharan Africa: A Prospective Cohort Study. Clin Infect Dis 55: 242-250.
- Munseri PJ, Talbot EA, Bakari M, Matee M, Teixeira JP, et al. (2011) The Bacteraemia of Disseminated Tuberculosis among HIV-infected Patients with Prolonged Fever in Tanzania. Scand J Infect Dis 43: 696-701.
- Jacob ST, Pavlinac PB, Nakiyingi L, Banura P, et al. (2013)Mycobacterium Tuberculosis Bacteremia in a Cohort of HIV-infected Patients Hospitalized with Severe Sepsis in Uganda-High Frequency, Low Clinical Sand Derivation of a Clinical Prediction Score. PLOS One8: e70305.
- Archibalk LK, den Dulk MO, Pallangyo KJ and Reller LB (1998) Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Inf Dis 26: 290-296.
- Brisson-Noel A, Aznar C , Chureau C, Nguyen S, Pierre C (1991) Diagnosis of tuberculosis by DNA amplification in clinical practice evaluation. Lancet 338: 364-366.
- Folgueira L, Delgado R, Palenque E, Aguado JM and Noriega AR (1996) Rapid Diagnosis of Mycobacterium tuberculosis Bacteremia by PCR. J Clin Microbiol 34: 512-515.
- Narayanan S, Das S, Garg R, Hari L, Rao VB, et al. (2002) Molecular epidemiology of tuberculosis in a rural area of high prevalence in South India: implications for disease control and prevention. J Clin Microbiol 40: 4785-4788.
- Lopez-Alvarez R, Badillo-Lopez C, Cerna-Cortes JF, Castillo-Ramirez I, Rivera-Gutierrez S, et al. (2010) First insights into the genetic diversity of Mycobacterium tuberculosis isolates from HIV-infected Mexican patients and mutations causing multi drug resistance. BMC Microbiol.10: 82.
- Daum LT, Rodriguez JD, Worthy SA, Peters RPH, Fourie PB, et al. (2015) Development of a multiplex real-time PCR assay for detection of Mycobacterium tuberculosis from sputum collected in Prime Store MTM®. Presented at The 46th Union World Conference on Lung Health, December 2015.
- Clough MC. The cultivation of tubercle bacilli from the circulation blood in military tuberculosis. Am Rev Tuberc 1: 598-621.
- Munseri PJ, Talbot EA, Bakari M, Matee M, Teixeira JP, et al. (2011) The bacteraemia of disseminated tuberculosis among HIV-infected patients with prolong fever in Tanzania. Scand J Infect Dis 43: 696-701.
- Crump JA, Morrissey AB, Ramadhani HO, Njau BN, Maro VP, et al. (2011) Controlled comparison of BacT/alert MB system, manual myco/F lytic procedure, and isolator 10 system for diagnosis of Mycobacterium tuberculosis bacteremia. J Clin Microbiol 49: 3054-3057.
- Feasey NA, Banada PP, Howson W, Sloan DJ, Mdolo A, et al. (2013) Evaluation of Xpert MTB/RIF for Detection of Tuberculosis from Blood Samples of HIV-Infected Adults Confirms Mycobacterium tuberculosis Bacteremia as an Indicator of Poor Prognosis. J Clin Micro 51: 2311-2316.
- Daum LT, Peters RP, Fourie PB, Jonkman K, Worthy SA, et al. (2015) Molecular Detection of Mycobacterium Tuberculosis from Sputum Transported in Prime Store® from Rural Settings. Int J Tuberc Lung Dis 19: 552-557.
- Daum LT, Choi Y, Worthy SA, Rodriguez JD, Chambers JP, et al. (2014) Molecular transport medium for collection, inactivation, transport, and detection of Mycobacterium tuberculosis. Int J Tuberc Lung Dis 18: 847-849.
- Omar SV, Peters RP, Ismail NA, Dreyer AW, Said HM, et al. (2015) Laboratory evaluation of a specimen transport medium for downstream molecular processing of sputum samples to detect Mycobacterium tuberculosis. J Microbiol Methods 117: 57-63.
- Daum LT, Fourie PB, Peters RP, Rodriguez JD, Worthy SA, et al. (2016) Xpert® MTB/RIF detection of Mycobacterium tuberculosisfrom sputum collected in molecular transport medium. Int J Tuberc Lung Dis 20: 1118-1124.