Zheng-qin Gao1*, and Bing-fei Yue1
1National Institutes for Food and Drug Control (NIFDC), No.2 Temple of Heaven, Dongcheng District, Beijing 100050, China
Corresponding author
Dr. Zheng-qin Gao, National Institutes for Food and Drug Control (NIFDC), No.2 Temple of Heaven, Dongcheng District, Beijing 100050, China, E-mail: gaozhengqin@126.com
Received Date: 22ndOctober 2014
Accepted Date: 12th November 2014
Published Date: 16th November 2014
Citation
Gao ZQ, Yue BF (2014) Rapid Detection of Ectromelia Virus by A Taqman-MGB Probe-Based Fluorescence Quantitative Real-Time PCR Assay. Enliven: Microb Microbial Tech 1(1): 002.
Copyright
@ 2014 Dr. Zheng-qin Gao. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background
Ectromelia virus (ECTV) is an important pathogen that could cause the acute lethal viral disease known as mousepox. This disease has severe epidemic feature with high mortality and significant harm. In order to prevent and control the occurrence of mousepox, it is essential to develop a fast, sensitive diagnostic tool for rapid and specific detection of infectious Ectromelia virus. The aim of this study was to develop a TaqMan-minor groove binder (TaqMan -MGB) probe-based fluorescence quantitative real-time polymerase chain reaction (qPCR) assay for rapid detection of Ectromelia virus.
Methods
Plasmid containing the sequence of HA gene (168155-168239 nt) was constructed as ECTV-DNA standard for quantitative analysis. In this study, a set of reliable and specific qPCR primers and a TaqMan MGB probe were used to detect Ectromelia virus. The interested sequence contained in the plasmid and in clinical specimens was quantitatively measured.
Results
The TaqMan-MGB probe qPCR assay was shown to be 100% specific to test a panel of seventeen organisms consisting of ECTV, other orthopoxvirus species, non-orthopoxvirus species, bacteria, fungi, parasites and cells. The technology was demonstrated to be highly sensitive, allowing a precise ECTV DNA quantitation over a range of ten orders of magnitude (from 100 to 1010 copies of standard DNA), and the limit of detection (LOD) of the assay was determined to be 3 copies of target DNA. The reproducibility of standard curve was excellent as the variation coefficient (%CV) for each point was less than 3%. Measurements of linearity such as R2, S, y, and Eff(%) did not vary significantly among the test (%CV<3%) suggesting high accuracy. The TaqMan MGB probe qPCR assay was successfully applied to quantifiable detection of viral genomic load in clinical specimens, and confirmed by using ELISA, conventional PCR and sequence analysis. The assay described in this report generates complete result in 2 h and can be used as a rapid diagnostic tool.
Conclusion
Our study demonstrated that this new TaqMan-MGB probe qPCR assay is an accurate, rapid and reliable method that can be used for the quantitative detection of ECTV, especially allowing the detection of low loading of ECTV in clinical specimens. It also could be applied to quantifiable detection of viral loading in animal origin products and biological products, food and drug safety inspection, environmental monitoring and epidemiology investigation. It is the first report to describe a TaqMan-MGB probe qPCR for specific diagnosis of mousepox infection.
Keywords
Ectromelia virus (ECTV); TaqMan-minor groove binder (TaqMan-MGB) probe; Fluorescence quantitative real-time PCR (qPCR) assay; Rapid detection
Background
Ectromelia virus belongs to the family Poxviridae, the sub-family Chordopoxvirinae, the genus of Orthopoxvirus (the current classification of the International Committee on Taxonomy of Viruses (ICTV) (http://ictvonline.org/index.asp), and it is the enveloped DNA virus which contains a covalently closed, double-stranded DNA genome of approximately 200 kbp [1-3].
Ectromelia virus is an important pathogen that could cause the acute lethal viral disease known as mousepox. Its clinical manifestations include swelling of limbs, tail and head, ulceration, necrosis and even toes shedding, so it is also known as Ectromelia [4-6]. The disease has severe epidemic feature with high mortality and significant harm. In 1930, Marchal first reported this disease mainly characterized as acute hepatic necrosis, pox spots or scattered stealth infection in mice. Subsequently, many countries in Europe, North America and Asia also reported the prevalence of this disease [7-11]. In recent years, researchers in United States have successively reported that the occurrence of the disease in USA was related to domestic and foreign imported mice infected by Ectromelia virus and murine origin products contaminated by Ectromelia virus [12,13].
In order to prevent and control the occurrence of mousepox, it is essential to develop a rapid, sensitive diagnostic tool for rapid and specific detection of infectious Ectromelia virus is essential. The traditional diagnosis of mousepox is based on clinical manifestations, virus isolation, electron microscopy, animal inoculation and serologic test [14-18]. The identification of clinical manifestations requires laboratory confirmation, the isolation of live Ectromelia virus in cell cultures is technically difficult and time-consuming, Electronic microscopy is not a convenient method for routine purposes, and serological assays are lack of sensitivity and specificity, thus they are not suitable for detection of Ectromelia virus in the environment, cell culture medium, food, drug and biological products from animal origin [19-22]. In order to overcome these inconveniences, as an alternative approach, Sequencing/PCR-RFLP (restriction fragment length polymorphism analysis) has been successfully used for identify Ectromelia virus [23,11], as the operation requires handling of amplified target DNA, which may be contaminated in workspace and is relatively time intensive, it is only suitable for species or strain identification.
Compared with conventional PCR-based virus detection assay, real-time quantitative PCR-based assay combines amplification with detection of target DNA in one vessel, thereby eliminating any time-consuming post-PCR procedures and potentially limiting possible contamination. Detection systems available for real-time PCR can also be nonspecific where the intercalating dyes (e.g., SYBR green I dye) generate fluorescence bonding to PCR fragments [24,25]. Due to the high sensitivity of minor groove binder (MGB) DNA probe to nucleotide mis-matches, real-time fluorescence quantitative PCR (qPCR) using MGB probe has been extensively used in detection of different viruses, including species of orthopoxviruses, such as monkeypox virus [26] and smallpox virus [27-29].
To date, there is no report on a rapid and sensitive real-time PCR assay for specific detection and quantification of Ectromelia virus. Therefore, establishing a rapid, specific and sensitive real-time PCR detection method would be useful for quarantine, epidemiological, research, and mousepox control. In the present study, a set of reliable and specific qPCR primers and a TaqMan MGB probe were used to detect Ectromelia virus. TaqMan MGB probe is a new class of TaqMan probe conjugated with a MGB at 3' end. Compared with TaqMan probe, TaqMan MGB probe has several advantages, such as high specificity and sensitivity, remarkable accuracy and reproducibility [30]. Here, for the first time, we report the development and application of the TaqMan MGB probe qPCR assay for rapid detection of Ectromelia virus.
Materials and Methods
Ninety-nine serum specimens from mice-infected with Ectromelia virus (6 positive specimens) and healthy controls (93 specimens) used for this study were obtained from National Institutes for Food and Drug Control (NIFDC). The clinical observation of mice infected naturally with Ectromelia virus (C57BL/6 mice no.1-no.2 and KM mice no. 3-no.6): ruffled hair coat and hunched appearance, action reduced, mouth, ears, neck, perianal and tail skin ulcers, blood oozing, crusting, perineum small nodular swelling ulceration, greyish yellow cheese-like material can be extruded. Necropsy observation and histopathological changes in all six mice: liver enlargement with gray necrotic foci, splenomegaly associated with gray necrotic foci, swollen lymph nodes, intestinal bleeding. The collection of specimens for this work was conducted in accordance with the guidelines of the Laboratory Animal Welfare Ethical Review Committee of NIFDC in Beijing, China.
Serum Ectromelia virus antibody was measured by commercially available Mouse Ectromelia virus ELISA kit (NIFDC, Beijing, China) according to the manufacturer’s instructions. The DNA was extracted from sera by using QIAamp DNA mini kit (QIAGEN, Hilden, Germany) as per the manufacturer’s protocol. The DNA specimen was quantified using the absorbance at 260nm, 280nm by BioSpec-mini (Shimadzu, Japan) and stored at –70s till use.
A panel of seventeen organisms consisting of Ectromelia virus, Vaccinia virus, Japanese encephalitis virus, Lymphocytic choriomeningitis virus, Encephalomyocarditis virus, Encephalomyelitis virus, Clostridium piliforme, Campylobacter jejuni, Helicobacter pylori, Helicobacter hepaticus, Mycoplasma pneumoniae, Pasteurella pneumotropica, Cilia-associated respiratory bacillus, Candida albicans, Toxoplasma gondii, Giardia lamblia and BHK cells (Baby hamster kidney cells) were stored in our laboratory at –70s for later use.
Ectromelia virus was cultured in BHK cells and its titer was determined by plaque assay using the same cell lines. Ectromelia virus was diluted 1.0×108 plaque-forming units (PFU)/mL per tube. DNA was extracted by using QIAamp DNA mini kit in accordance with the manufacturer’s instructions and used in the PCR assay.
A fragment of 846 bp which contained the hemagglutinin (HA) gene was PCR amplified from Ectromelia virus genomic DNA by using the forward primer GZQECTV-HAF (5'-ATGGCACGATTGTCAATACT-3') and reverse primer GZQECTV-HAR (5'-CTAGACTTTGTTCTCTGT-3'). The fragment was cloned into the pMD18-T vector (TaKaRa, Dalian, China) according to the manufacturer’s protocol. Briefly, the target HA gene was amplified by high-fidelity PCR from Ectromelia virus genomic DNA. DNA fragment was confirmed by agarose gel electrophoresis, recovered and purified by using Agarose Gel DNA Purification Kit (TaKaRa), and 2 µL (200 ng) of Insert DNA (purified DNA) was added to 1 µL (50 ng) of Vector DNA (pMD18-T). Mixture was incubated for 30 min at 16s, and full amount of ligation reaction liquid was used to transform competent JM109 Escherichia coli competent cell (TaKaRa) that was spread-plated on Luria-Bertani (LB) agar containing ampicillin (100 µg/mL) (Sigma, St. Louis, MO, USA), IPTG (isopropyl-β-D-thiogalactopyranoside) (0.5 mM) (Sigma), and X-Gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) (40 µg/ml) (Sigma). Positive clone was detected by restriction enzyme digestion, PCR amplification of the inserted DNA, and sequencing. The recombination plasmid DNA was extracted by using Plasmid Purification Kit (TaKaRa) and measured by optical density at 260 nm. The pMD18-T clone containing HA insert was sequenced on Prism 3100 genetic analyzer (Life Technologies, Foster City, CA, USA). Approximately 100 ng of recombination plasmid DNA was used as the template in BigDye terminator cycle ready reactions (Life Technologies) in accordance with the manufacturer’s instructions. Clones containing HA gene insert was sequenced with sequencing primers M13-47: 5'-CGCCAG GGTTTTCCCAGTCACGAC-3'; RV-M: 5'-GAGCGGATAACAATTTCACACAGG-3'; pMD18 F: 5'-GCCTCTTCGCTATTACGCCA-3'; pMD18 R: 5'-CACTCATTAGGCACCCCAGG-3'. DNA sequence comparisons were made using DNASTAR alignment software (DNASTAR, Inc., Madison, Wis).
To establish the standard curve of the TaqMan MGB probe qPCR assay for Ectromelia virus, the copy number was calculated for the recombination plasmid carrying one copy of the specific HA gene. The gene copy number of HA was calculated as follows: Copies/mL = 6.02 × 1023 (Avogadro’s number) × DNA (concentration g/mL) / (MW g/mol); MW = genome length × 660 dalton/bp. Knowing the number of plasmid molecules with the target per tube, a series of dilutions of Ectromelia virus recombination plasmid were used as the internal controls and the analytical standards to generate TaqMan MGB probe qPCR standard curves.
The qPCR primers (GZQECTV-HATF, 5'-CGACTCCGGAACCAATTACTGATAA-3'; GZQECTV-HATR, 5'-ACTCCAGATGATGTACTTACTGTAGTGT-3') and TaqMan MGB probe (GZQECTV-HATMP, 5'- FAM-TACAACAGTGTCTGTGACT-MGB-NFQ-3') were designed using “Primer Express” Software (version 3.0) [36], The TaqMan MGB probe was labeled with the fluorescent reporter dye FAM (6-carboxyfluorescein) at the 5' end and with the MGB and a non-fluorescent quencher (NFQ) at the 3' end. The TaqMan MGB probe and qPCR primers were commercially synthesized by Life Technologies (Carlsbad, California, USA). After design, the primers (GZQECTV-HATF; GZQECTV-HATR) and TaqMan MGB probe (GZQECTV-HATMP) were again checked using the National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLAST) (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to avoid any cross-reactivity with other species.
Amplification reaction contained 10 µL of 2 × TaqMan Universal Master Mix (Life Technologies, USA), 1.0 µL each of 500 nM concentrations of the primers (GZQECTV-HATF and GZQECTV-HATR) and 250 nM concentrations of the probes (GZQECTV-HATMP), 1.0 µL of template DNA, and nuclease-free water to a final volume of 20 µL. All DNA specimens were assayed in MicroAmp optical 96-well plates (Life Technologies, California, USA), closed with MicroAmp optical caps, were run in triplicates and all assays were repeated at least twice. The thermal profile consisted of 50s for 2 min, followed by 95s for 10 min and 40 cycles of 95s for 15s and 60s for 1 min. Fluorescence was measured once per cycle at the end of the 60s segment. The TaqMan MGB-probe qPCR assay was performed on 7500 Fast Real-time PCR System (Life Technologies, California, USA), and the data generated were analyzed with the SDS Software in the instrument.
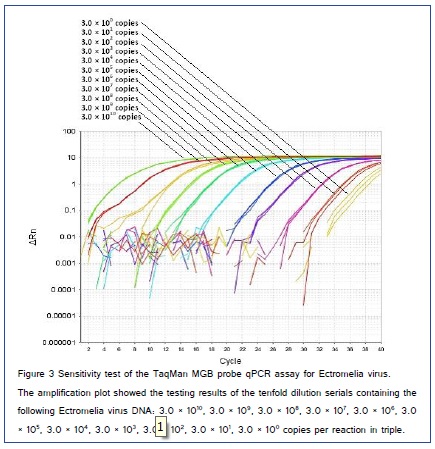
The TaqMan MGB probe qPCR assay with the primers and probe sets were performed with template from Ectromelia virus and negative-control specimens to demonstrate specificity for Ectromelia virus. To determine the efficiency of the TaqMan MGB probe qPCR, each reaction contained a serially tenfold diluted Ectromelia virus plasmid DNA as the internal control. This control was present at a predetermined concentration (100 to 1010 copies per reaction) and acted as plasmid calibrant to determine the efficiency of the TaqMan MGB-probe qPCR and provide quantitative information. Measurements of Ectromelia virus DNA in unknown specimens were made by interpolation from a standard curve generated with the standards Ectromelia virus DNA, which was amplified in the same qPCR run. In order to construct a standard curve and to determine the limit of detection of this sensitive assay, tenfold serial dilutions of Ectromelia virus DNA standard (100 ng, 10 ng, 1 ng, 100 pg, 10 pg, 1 pg, 100 fg, 10 fg, 1 fg, 100 ag, and 10 ag, approximately 3.0 × 1010 to 3.0 × 100 copies/tube, respectively) were used in our study. The calibration curve was constructed by plotting the Ct values against known serial dilutions of the standards Ectromelia virus DNA. Results were analyzed using an SDS 1.9 sequence detection system (Life Technologies, California, USA) after manual adjustment of the baseline and fluorescence threshold.
The developed analytical standard was used to calculate the intra and inter-assay reproducibility of quantification for the specific primers set and probe. Mean Ct values, standard deviation (SD) and coefficient of variation (CV) were calculated from the data obtained in three replicates of each standard dilution for the intra-assay reproducibility, and in three TaqMan MGB-probe qPCR assays consisted of three replicates each (six total) for the inter-assay reproducibility. CV was calculated as SD/Mean Ct * 100%.
These DNA extracted from specimens were used to detect and quantify Ectromelia virus by standardized TaqMan MGB-probe qPCR assay as described above. The quantification cycle (Cq), previously known as the Ct values were compared with the standard curve data to calculate the quantity of Ectromelia virus DNA in each specimen.
Results and Discussion
Online sequence similarity analysis (http://www.ncbi.nlm.nih.gov/ BLAST/) showed that the Ectromelia virus hemagglutinin (HA) gene sequence cloned (Figure 1), for which the qPCR primers and TaqMan MGB probe were designed, showed no similarity with other sequences but had a 100% sequence identity with the target Ectromelia virus HA gene in NCBI (Table 1).
| Name | Sequence 5'→3' | Location (GenBank no.) | Product |
| GZQECTV-HATF | CGACTCCGGAACCAATTACTGATAA | 168155-168179 (JQ410350.1) | 85 bp |
| GZQECTV-HATR | ACTCCAGATGATGTACTTACTGTAGTGT | 168212-168239 (JQ410350.1) | |
| GZQECTV-HATMP | CTGTGACTGTATGATCTTC-MGB-NFQ | 168181-168199 (JQ410350.1) |
Table 1 Sequences of qPCR primers and TaqMan-MGB probe used in our study
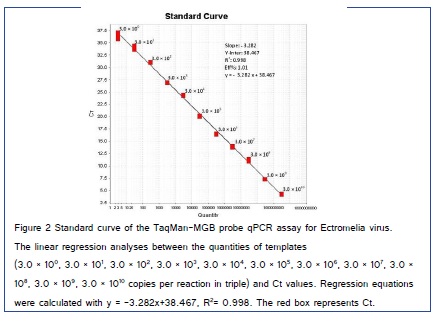
Rn is the fluorescence of the reporter dye divided by the fluorescence of a passive reference dye. In other words, Rn is the reporter signal normalized to the fluorescence signal of ROX™. ΔRn is Rn minus the baseline, graphed here versus the cycle of PCR. Amplification plot shows the Log (ΔRn) graphed versus cycle. Correlation coefficient (R2), slope (S), and efficiency (Eff% = 10 (1/S) − 1) of amplification were calculated to assess the linear range and reliability of the TaqMan MGB probe qPCR [31]. By the equation of the linear regression line (y = a x + b) (a was the slope (S) and b was the intercept (Y-Inter)), the copy number can be calculated in specimens. The formula for the copy number was inferred from the equation: copy number = λ × 10(Ct−b)/a. In this study, λ was a fixed value as 3.0 × 1010 for the dynamic range of Ectromelia virus which was obtained by the copy number for each serial dilution.
In generated standard curves of the TaqMan MGB probe qPCR assay for Ectromelia virus, linearity between the cycle threshold (Ct) value and target concentrations were observed ten orders of magnitude in tenfold serial dilutions of Ectromelia virus standard DNA in triplicate. A linear regression analysis (R2 = 0.998; Eff% = 1.01), which revealed a linear relationship between the quantities of templates and Ct values, was performed with ΔRn and Ct, and the results were shown in (Figure 2), which demonstrated that the TaqMan MGB probe qPCR protocol was feasible to Ectromelia virus pathogen quantification. The minimum that could be detected was 3 copies per reaction as indicated in the following TaqMan MGB probe qPCR assays (Figure 3). The data depicting mean Ct, standard deviation (SD), and CV for the primers and probe set selected for the Ectromelia virus TaqMan MGB probe qPCR array with each standard concentration are shown in Table 2. The reproducibility of standard curve was excellent as the variation coefficient (%CV) for each point was below 3%. Measurements of linearity such as R2, S, y, and Eff(%) did not vary significantly among the test (%CV?3%). This result showed that the TaqMan MGB probe qPCR assay has good stability and reproducibility. Therefore, this TaqMan MGB probe qPCR could be expanded to estimate gene copy number and by extension, percent viral loading in clinical specimens.
| Serial dilution (copies/tube) (n = 18) | Standard curve result for a: | |
| Ct ± SD | % CV | |
| 3.0 × 106 | 16.448±0.085 | 0.52 |
| 3.0 × 105 | 20.033±0.078 | 0.39 |
| 3.0 × 104 | 24.292±0.101 | 0.42 |
| 3.0 × 103 | 26.899±0.076 | 0.28 |
| Other parameters (n =6) | Mean ± SD | % CV |
| Slope | 3.282±0.062 | 1.89 |
| Y-Inter | 38.467±0.419 | 1.09 |
| R2 | 0.998±0.001 | 0.1 |
| Eff% | 101.676±2.756 | 2.71 |
Table 2 Reproducibility of the TaqMan MGB probe qPCR assay for Ectromelia virus
aStandard curves for tenfold serial dilutions were Ectromelia virus standard DNA. The DNA control was diluted in nuclease-free water to cover a linear range of ten orders of magnitude. Each curve was run in triplicate in six independent assays. Reproducibility was assessed by computing the coefficient of variation (%CV) among the mean values in six independent assays. The efficiency of the standard curves was calculated based on the slope from six independent experiments.
Amplification of the Ectromelia virus was observed from fourteen cycles onwards as indicated by an increase in the fluorescence intensity. The fluorescence intensity values for Vaccinia virus, Japanese encephalitis virus, Lymphocytic choriomeningitis virus, Encephalomyocarditis virus, Encephalomyelitis virus, Clostridium piliforme, Campylobacter jejuni, Helicobacter pylori, Helicobacter hepaticus, Mycoplasma pneumoniae, Pasteurella pneumotropica, Cilia-associated respiratory bacillus, Candida albicans, Toxoplasma gondii, Giardia lamblia and BHK cells remained at the base line similar to nuclease-free water indicating that the test successfully detected Ectromelia virus (Figure 4).
For evaluating the usefulness of TaqMan MGB probe qPCR for detecting Ectromelia virus in clinical specimens, 99 sera were tested by Mouse Ectromelia virus ELISA kit and TaqMan MGB probe qPCR. Except for these healthy controls (93 specimens), all 6 DNA specimens extracted from mice-infected with Ectromelia virus (6 positive specimens) were found to be positive by TaqMan MGB probe qPCR. All TaqMan MGB probe qPCR tested positive specimens were also positive by Mouse Ectromelia virus ELISA kit. The Ct values for the 6 positive specimens were low (16.0, 14.6, 18.3, 11.9, 12.0, 8.9; mean 13.6). All six positive specimens from two C57BL/6 mice (no. 1 and no. 2) and four KM mice (no.3 to no. 6) showed evidence of Ectromelia virus DNA at comparatively high concentrations (average DNA concentration of positive specimen 3.0 × 107 copies) for those mouse species. The results of testing 99 clinical specimens by conventional PCR, and TaqMan MGB probe qPCR reveal that 6 (6.1%) were positive in both assays. These results indicate that both assays are adequate for the detection of Ectromelia virus from clinical specimens (liver, spleen, intestines, lymph node, blood, serum).
To confirm the amplification of Ectromelia virus DNA in the six positive specimens and to determine the identity of observed bands in the initial reaction, DNA fragment was confirmed by agarose gel electrophoresis. PCR products were purified via gel excision, recovered and purified by using Agarose Gel DNA Purification Kit (TaKaRa, Dalian, China) following the manufacturer’s protocol. Purified PCR products were direct sequenced in both directions using the appropriate PCR primers (TaKaRa) and the sequences were edited and aligned in Sequencher v.4.2.2 (GeneCodes, Ann Arbor, MI, USA), and identified using BLASTN (www.ncbi.nlm.nih.gov). For the six amplicons from positive specimens that were sequenced, all were 100% identical to the published target sequence for the Ectromelia virus genomic DNA HA gene (GenBank: JQ410350.1; KJ563295.1; AF375091.1; AF375092.1; AY902306.2; AY902304.2; AY902303.2; AY902302.2; AF012825.2; DQ003024.1; Z99053.1; AF012825.2) (data not shown).
None of negative control specimens tested by the assay showed positive amplification, suggesting that the test was 100% specific for the detection of Ectromelia virus [number of negative specimens/(number of negative specimens + number of false positive specimens).The TaqMan MGB probe qPCR assay could detect Ectromelia virus positive specimen indicating that the test was 100% sensitive in detecting Ectromelia virus [(number of positive specimens/(number of positive specimens + number of false negative specimens)] (Table 3).
| Specimen type (total no.) | Results of TaqMan MGB probe qPCR for | |
| No. (%) of positive specimens | Ct ± SD | |
| Ectromelia virus (1) | 1(100) | 15.8±0.04 |
| Vaccinia virus (1), Japanese encephalitis virus (1), Lymphocytic choriomeningitis virus (1), Encephalomyocarditis virus (1), Encephalomyelitis virus (1), Clostridium piliforme (1), Campylobacter jejuni (1), Helicobacter pylori (1), Helicobactel hepaticus (1) , Mycoplasma pneumonia (1), Pasteurella pneumotropica (1), Cilia-associated respiratory bacillus (1), Candida albicans (1), Toxoplasma gondii (1), Giardia lamblia (1), BHK cell (1) | 0 (0) | ?40 |
| Serum | ||
| Infected (6) | 6(100) | 13.6 ±0.11 |
| Healthy (93) | 0 (0) | ?40 |
Table 3 TaqMan MGB probe qPCR for detecting Ectromelia virus in specimens
In this study a new TaqMan MGB probe qPCR assay was developed and evaluated for detection of Ectromelia virus in clinical specimens. The assay described in this report generates complete result in 2 h and can be used as a rapid diagnostic tool. Efforts are underway to test the utility of this TaqMan MGB probe qPCR assay using specimens with more diverse biological origin and pathogen content (data not shown). This method may also be effective for screening animal origin products, an important consideration in the face of the reports of transmission-associated infections described above.
Ectromelia virus is a member of the family Poxviridae, the genus of Orthopoxvirus. The orthopoxviruses (OPV) are allocated into 11 species, 8 Eurasian-African species (variola virus (also known as smallpox virus), monkeypox virus, vaccinia virus (including rabbitpox and horsepox), cowpox virus, camelpox virus, Ectromelia virus (also known as ectromelia), taterapox, and Uasin Gishu disease viruses) and 3 North American species (raccoon poxvirus, volepox virus, and skunkpox virus) [32]. Within the OPV genus, Ectromelia virus, Monkeypox virus, and Cowpox virus strain Brighton Red (BR) do not group closely with any other OPV [33]. The hemagglutinin (HA) gene is conserved among different species of orthopoxviruses [34,35]. To date, there is no study reporting the use of TaqMan MGB probe qPCR for the detection of Ectromelia virus. Here, we developed a TaqMan MGB probe qPCR assay that is rapid, specific, and sensitive for detecting Ectromelia virus. In this work, the qPCR assay was based on the TaqMan MGB probe that incorporated a 5' reporter dye and a 3' NFQ. The NFQ offers the advantage of lower background signal, which results in better precision in quantitation, stabilizing the hybridization of the probe with single-stranded DNA targets and leading to improved specificity over conventional TaqMan probes. Based on the data we have so far, this is indeed the first report of a TaqMan MGB probe qPCR-based Ectromelia virus detection method. The assay can specifically differentiate Ectromelia virus from other species of the orthopoxviruses. In our study, quantitative PCR primers and TaqMan MGB probe were designed very specific to detect the specific species HA region of Ectromelia virus. The specificity of the assay by showing successfully detection of Ectromelia virus, but not Vaccinia virus, Japanese encephalitis virus, Lymphocytic choriomeningitis virus, Encephalomyocarditis virus, Encephalomyelitis virus, Clostridium piliforme, Campylobacter jejuni, Helicobacter pylori, Helicobactel hepaticus, Mycoplasma pneumoniae, Pasteurella pneumotropica, Cilia-associated respiratory bacillus, Candida albicans, Toxoplasma gondii, Giardia lamblia, and BHK cells nucleic acid. We also used other species of orthopoxviruses as negative controls and show the primers and probe used in this assay does not cross react with them.
We have demonstrated that this TaqMan MGB probe qPCR assay is highly effective as a diagnostic tool for the detection of Ectromelia virus in clinical specimens. This assay is 100% specific to all specimens tested. We concluded that this assay permits speciation of the infecting Ectromelia virus. We also provided evidence that the assay can detect gene copy numbers far below those which would be expected in a clinical infection. The sensitivity has been determined to be 3 copies Ectromelia virus per reaction. With the inclusion of a set of specimens to generate a calibration curve, the excellent linearity of response also permits calculation of the percent Ectromelia virus loading. Global trade is the main cause of introduction of alien species, allowing long distance dispersal of some pathogens [12,13]. For these reasons, the development of highly accurate and reliable molecular detection technique could help in avoiding the infection of the pathogen. For effective early surveillance of this pathogen and to prevent its diffusion into new population, there is a real need for rapid, simple, and robust detection method. The use of serological testing method combined with effective routine molecular detection tool can provide more accurate forecasts of the risk of pathogen spread and be helpful to the management of the disease.
Conclusion
The TaqMan MGB probe qPCR assay described here and validated as a clinical assay is a rapid and sensitive method for detection of Ectromelia virus and a valuable new tool for diagnostic laboratories. It also could be applied to Ectromelia virus detection for animal origin products and biological products, food and drug safety inspection, environmental monitoring and epidemiology investigation.
Competing Interests
The authors have declared that no competing interests exist.
Acknowledgements
We are grateful to Sheryl Acorda provided valuable comments on an earlier version of the manuscript. This work was supported by a grant from Youth Research Development Foundation of National Institutes for Food and Drug Control (No. 2010C5). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
ZQG carried out the molecular biological studies, participated in the sequence alignment and drafted the manuscript. BFY participated in the sequence alignment. ZQG participated in the design of the study and performed the statistical analysis. BFY conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.