Original Article
Authors:
Jiann-Tsyh Lin*, and Grace Q. Chen
Western Regional Research Center, Agricultural Research Service, U.S. Department of Agriculture, 800 Buchanan Street, Albany, California 94710, USA
Corresponding author
Jiann-Tsyh Lin, Western Regional Research Center, Agricultural Research Service, U.S. Department of Agriculture, 800 Buchanan Street, Albany, California 94710, USA Tel: 510-559-5764; Fax: 510-559-5768; E-mail: jiann.lin@ars.usda.gov Received Date: 05 August 2014; Accepted Date: 30 September 2014; Published Date: 1 October 2014
Citation
Lin JT, chen GC (2014) Quantification of the Molecular Species of Tetraacylglycerols in Lesquerella (Physaria fendleri) Oil by HPLC with ELSD and MS. Enliven: Bio Anal Techniques 1(2): 003
Copyright
@ 2014 Jiann-Tsyh Lin. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, that permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Thirteen molecular species of tetraacylglycerols in the seed oil of Physaria fendleri were recently identified. We report here the quantification of the molecular species of these tetraacylglycerols using HPLC with evaporative light scattering detector and the MS of the HPLC fractions. The ion signal intensities of MS1 from the molecular species of acylglycerols with different m/z in HPLC fractions were used to estimate the ratios of the molecular species. The ratios of the molecular species of acylglycerols with the same mass were estimated by the total ion signal intensities of the fragment ions of MS2, [M + Li ? FA]+ and [M + Li ? FA-FA]+. The content of tetraacylglycerols was about 1%. The highest contents of the molecular species of tetraacylglycerols were from those containing one normal FA (non-hydroxylated fatty acid) and they were LsLsLsO (0.25%), LsLsLsL (0.24%), LsLsLsLn (0.21%) and LsLs-OH20:2-O (0.19%). The contents of the tetraacylglycerols containing two normal FA were lower than those containing one normal FA. Among them the highest were LsLsOL (0.10%), LsLsOO (0.06%) and LsLsOLn (0.06%).
Keywords
Quantification; Tetraacylglycerols; Lesquerella oil; Physaria fendleri; Mass spectrometry; HPLC
Introduction
Ricinoleic acid (R, OH1218:19) has many industrial uses such as the manufacture of biodegradable and renewable plastics, nylon, plasticizers, lubricants, cosmetics, paints and surfactants [1]. Ricinoleate containing a hydroxyl group is the major fatty acid (FA, 90%) in castor oil [2]. Tetraacylglycerol (an acylglycerol estolide) contains an acyl chain attached to the hydroxyl group of another acyl chain attached to the glycerol backbone. We have earlier positively identified a tetraacylglycerol for the first time in a natural source, (12-ricinoleoylricinoleoyl)-diricinoleoyl-glycerol (RRRR), in castor oil using electrospray ionization tandem mass spectrometry (ESI-MS2) [3]. For the structure of RRRR, [3]. We have also for the first time shown the biosynthesis of estolide by incorporating radiolabeled triricinolein (RRR) into tetraricinolein (RRRR) in castor microsomes [3]. The content of RRRR in castor oil was about 0.5% [4]. Some minor tetraacylglycerols, RRRL, RRRO, RRRP, RRRS and RRRLn, were later identified in castor oil [5]. Abbreviations of FA as the FA constituents of acylglycerols (AG) are given at the appendix a, (Table 1). In addition, we have identified 12 molecular species of diacylglycerols (DAG) and 53 molecular species of triacylglycerols (TAG) containing mono-, di-, and trihydroxy FA [4-9] as well as 12 molecular species of TAG containing three normal FA (non-hydroxylated) [5] in castor oil. The structures of seven hydroxy FA in castor oil were proposed [5-8]. The molecular species of some AG in castor oil have been quantified by HPLC with evaporative light scattering detector (ELSD) [4]. The content of RRR in castor oil was about 70% [4].
| Tetraacylglycerolsa (HPLC fraction # ) |
m/z of [M + Li]+ | Contents (%)b |
|---|---|---|
| LsLsLn (55) | 1,283.90 | 0.21 ± 0.06 |
| LsLsLsL (57, 58) | 1,285.90 | 0.24 ± 0.09 |
| LsLs-OH20:2-O (57, 58) | 1,285.90 | 0.19 ± 0.06 |
| LsLsLsO (59, 60) | 1,287.90 | 0.25 ± 0.00 |
| LsLsLnLn (63) | 1,235.90 | 0.02 ± 0.01 |
| LsLsLLn (66) | 1,237.90 | 0.04 ± 0.01 |
| LsLsOLn (68) | 1,239.90 | 0.06 ± 0.02 |
| LsLsLL (68) | 1,239.90 | 0.02 ± 0.01 |
| LsLsOL (70) | 1,241.90 | 0.10 ± 0.01 |
| LsLsOP (72, 73) | 1,217.90 | 0.02 ± 0.01 |
| LsLsOO (73) | 1,243.90 | 0.06 ± 0.00 |
| LsLsLS (74) | 1,243.90 | 0.03 ± 0.00 |
| LsLsOS (76) | 1,245.90 | 0.02 ± 0.00 |
a, abbreviations: Ls- lesquerolic acid; S- stearic acid; O- oleic acid;
L- linoleic acid; Ln- linolenic acid; P-palmitic acid; OH20:2,
monohydroxy group on 20 carbon atoms fatty acid with 2 double bonds.
b, contents are presented as average ± standard deviation of triplicate measurements.
Table 1: The contents (%) of tetraacylglycerols in the seed oil of Physaria fendleri
Lesquerolic acid (Ls, OH1420:111), a C20 homolog of ricinoleate, is the major FA (56.5%) in lesquerella (Physaria fendleri) oil [10]. Lesquerolate can also be used for industry similar to those of ricinoleate. We have recently identified ten molecular species of DAG, 74 molecular species of TAG and 13 molecular species of tetraacylglycerols containing mono- and dihydroxy FA as well as 20 molecular species of TAG containing three normal FA in lesquerella oil [11,12]. The structures of the four new hydroxy FA were proposed [11]. The quantification of these DAG and TAG in lesquerella oil has also been reported [13]. The contents of the molecular species of DAG in decreasing order were: LsLs (0.25%), LsLn (0.25%), LsO (0.24%), LsL (0.11%) and the contents of the molecular species of TAG in decreasing order were: LsLsO (31.3%), LsLsLn (24.9%), LsLsL (15.8%), LsL-OH20:2 (4.3%), LsO-OH20:2 (2.8%), and LsLn-OH20:2 (2.5%). The content of ten DAG combined was about 1% and 74 TAG was about 98%. Acylglycerol estolides (e.g., tetraacylglycerols) have different physical properties from those of TAG and can be used in industry similar to those of TAG for different physical properties such as viscosity and pour point improvers for lubricants. We have recently identified 13 molecular species of tetraacylglycerols in lesquerella oil [12]. We did not detect pentaacylglycerols in both castor and lesquerella oils [5,12]. We would like to report here the contents of the 13 molecular species of tetraacylglycerols in lesquerella oil here.
Experimental Procedure
The materials, HPLC with absorbance detector (205 nm) and MS used were the same as our recent report [12]. HPLC with ELSD was as our earlier report [4]. Molecular species of AG (DAG, TAG and tetraacylglycerols) were partially separated using a C18 analytical column (Gemini, 250 mm ? 4.6 mm, particle size 5?m, C18, Phenomenex, Torrance, CA, USA) with a linear gradient from 100% methanol to 100% 2-propanol in 40 min, at 1 mL/min flow rate. The quantification of the molecular species of AG in lesquerella oil was as our recent report [13] using HPLC with ELSD and MS of the HPLC fractions. MS2 was used to differentiate the molecular species of AG with the same mass. The ratios of the contents of the molecular species of tetraacylglycerols with the same mass in HPLC fractions were estimated as the ratios of the sums of the ion signal intensities (or relative abundances) of [M + Li − FA]+ and [M + Li − FA-FA]+ from one molecular species.
Results and Discussion
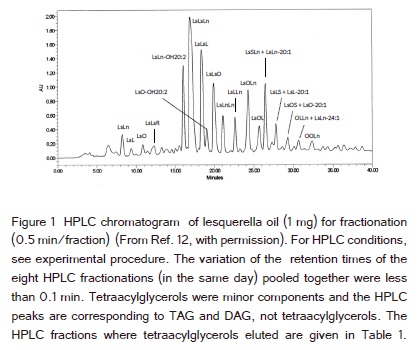
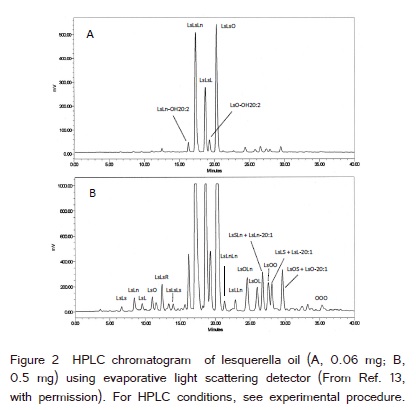
Lesquerella oil was fractionated (0.5 min/fraction) by HPLC as shown in (Figure 1). MS of each HPLC fraction was carried out using the lithium adducts of AG. Quantification of AG was based on HPLC with ELSD (Figure 2) and the MS ion signal intensities of the molecular species of AG in each HPLC fraction. About 98% of AG in lesquerella oil was TAG [13]. The contents of the molecular species of tetraacylglycerols were low and could not be shown as HPLC peaks in both (Figure 1 and 2) (quantification #1 of the three). Therefore HPLC with ELSD alone could not be used to estimate any content of the molecular species of tetraacylglycerol in lesquerella oil. The following are the examples of the quantifications of tetraacylglycerols using the combination of HPLC and MS.
(Figure 3) was the MS1 spectrum of the HPLC fraction #55 (Figure 1), (retention time 27.0−27.5 min). This HPLC fraction corresponded to the retention time 27.1−27.6 min in (Figure 2A) and 27.3−27.8 min in (Figure 2B). LsOO was mostly in this HPLC fraction [13]. The ion signal intensities of the known ions in (Figure 3) were 4.47 x 108 (m/z 935.8, LsOO), 1.97 x 108 (m/z 909.8, LsOP) and 7.15 x 107 (m/z 1284.0, LsLsLsLn). The peak area % of the four HPLC fractions from 27.3−29.3 min in (Figure 2B) was 3.25% (quantification #1 of the three). Five TAG and three tetraacylglycerols were recently identified in these four HPLC fractions including some AG with the same masses (lithium adducts) as: LsOP (m/z 909.8, HPLC fraction #55), LsOO (m/z 935.8, HPLC fraction #55, 56), LsLS (m/z 935.8, HPLC fraction #56, 57), LsL-20:1 (m/z 961.8, HPLC fraction #56, 57), LsLn-20:0 (m/z 961.8, HPLC fraction #58), LsLsLsLn (m/z 1283.9, HPLC fraction #55), LsLsLsL (m/z 1285.9, HPLC fraction #57, 58) and LsLs-OH20:2-O (m/z 1285.9, HPLC fraction #58) [11,12]. The ratio of the total ion signal intensities of each m/z in these four HPLC factions was assumed to be the ratio of these AG (with different m/z) in these four HPLC fractions. The total content of these five TAG and three tetraacylglycerols was 3.25% of lesquerella oil according to the area % of these four HPLC fractions in (Figure 2B). The contents of these AG including the AG with the same mass combined were estimated from the HPLC peak area % (3.25%, Figure 2B) and the ratio of the total ion signal intensities of each m/z in these four HPLC factions.
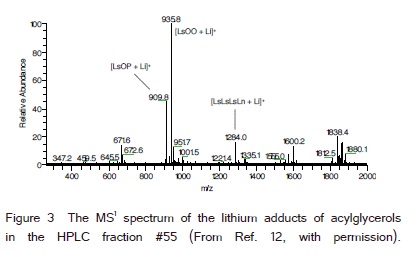
We have used MS2 fragmentation to estimate the ratios of TAG with the same masses [13]. The ratio of the total ion signal intensities of the three [M + Li − FA]+ from molecular ions with the same m/z was used to estimate the ratio of the TAG with the same mass, e.g., LsOO : LsLS and LsL-20:1 : LsLn-20:0 in these four HPLC fractions [13]. We have used the similar method to estimate the ratio of tetraacylglycerols with the same mass.
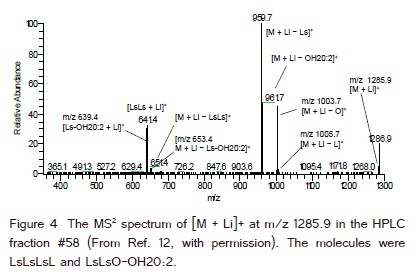
(Figure 4) is the MS2 spectrum of [M + Li]+ at m/z 1285.9 from the HPLC fraction #58 of lesquerella oil (retention time 28.5−29.0 min, (Figure 1). The ratio of the total ion signal intensities of the MS2 fragment ions, [M + Li − FA]+ and [M + Li − FA-FA]+, from molecular ions with the same m/z was used to estimate the ratio of the tetraacylglycerols with the same mass in a HPLC fraction. The tetraacylglycerols with the same mass were LsLsLsL and LsLsO-OH20:2 in (Figure 4). From the relative abundances (Y axis) of the fragment ions indicated in (Figure 4), the ratio of these two tetraacylglycerols in HPLC fraction #58 was estimated as (60 + 3 + 2) : (40 + 46 + 45 + 2 + 4) or 65 : 137. The 60 and 40 were from the relative abundance of [M + Li − Ls]+ at m/z 959.7 as 100%. Since there were five Ls in these two tetraacylglycerols, 20% represented each Ls, and 60 and 40 were distributed between these two tetraacylglycerols. The relative abundance of [M + Li − L]+ at m/z 1005.7 was 3%. The relative abundance of [M + Li − LsLs]+ at m/z 651.4 was 4%. Half of the 4% (2%) was equally distributed to these two tetraacylglycerols. The other relative abundances were 46% from [M + Li − OH20:2]+ at m/z 961.7, 45% from [M + Li − O]+ at m/z 1003.7, and 4% from [M + Li − Ls-OH20:2]+ at m/z 653.4. The HPLC fraction #57 also contained these two tetraacylglycerols with the predominance of LsLsLsL. The ratio of the total ion signal intensities (MS2) of the two tetraacylglycerolos in the HPLC fractions # 57 and 58 was used to estimate the contents of these two tetraacylglycerols from the content of tetraacylglycerols of m/z 1285.9 obtained by MS1 earlier.
The contents (%) of the molecular species of tetraacylglycerols in the seed oil of Physaria fendleri are listed in (Table 1). The total content of tetraacylglycerols was about 1.26% estimated from (Table 1). The content of TAG was about 98% and DAG was about 1% [13]. We did not detect pentaacylglycerol in the seed oil of Physaria fendleri [12] even though it was reported earlier [10,14]. The content of tetraacylglycerols could be increased for industrial uses. The highest contents of the molecular species of tetraacylglycerols (Table 1) were from those containing one normal FA (non-hydroxylated FA) and they were LsLsLsO (0.25%), LsLsLsL (0.24%), LsLsLsLn (0.21%) and LsLs-OH20:2-O (0.19%). The contents of the tetraacylglycerols containing two normal FA were lower than those containing one normal FA. Among them the highest were LsLsOL (0.10%), LsLsOO (0.06%) and LsLsOLn (0.06%). We did not detect tetraacylglycerol containing three normal FA in the seed oil of Physaria fendleri. The precursors of tetraacylglycerols containing one and two normal FA were proposed to be the TAG containing one normal FA, LsLsX (X = normal FA) [12]. We have recently proposed the biosynthetic pathways of tetraacylglycerols in castor [5] and Physaria fendleri [12].
Lipid quantification using ion signal intensities were reported [15-17]. We have recently used the HPLC with ELSD and MS (ion signal intensity) for the quantification of the molecular species of TAG and DAG [13]. Ionization efficiency of lipid depends on the polarity of molecule especially the difference of the lipid classes [18]. We compared the ion signal intensities of tetraacylglycerols and TAG in HPLC fractions in sequence and the polarities of these molecules were similar. We assumed that the ionization efficiencies of tetraacylglycerols and TAG in the HPLC fractions were similar. The ion signal intensities of the molecular species of TAG and tetraacylglycerols in the HPLC fractions in sequence were used to obtain the ratio of the molecular species in a HPLC peak.
We have recently identified many molecular species of TAG and DAG in lesquerella oil using the MS of HPLC fractions [11]. We have also identified later the molecular species of tetraacylglycerols, a group of naturally occurring acylglycerol estolides, in lesquerella oil using the MS of HPLC fractions [12]. Then, we developed a new method to quantify the molecular species of TAG and DAG in lesquerella oil using HPLC with ELSD and MS [13]. In this report we quantify the molecular species of tetraacylglycerols in lesquerella oil using HPLC with ELSD and MS. The MS2 ion signal intensities of fragment ions selected for the quantification of the molecular species of tetraacylglycerols with the same mass were different from those of TAG and DAG. The quantification of the molecular species of tetraacylglycerols in lesquerella oil helps to understand the biosynthesis of tetraacylglycerols in lesquerella oil and allows the future modification of the plant to produce tetraacylglycerols for industrial uses.
References
1. Gunstone FD, Alander J, Erhan SZ, McKeonTA, Lin JT (2007) Nonfood uses of oils and fats. In: Gunstone FD, Hardwood JL, Dijkstra AJ (eds) The Lipid Handbook, 3rd edn. Chapman & Hall, London: 591-635.
2. Achaya KT, Craig BM, Youngs CG (1964) The component fatty acids and glycerides of castor oil. J Am Oil Chem Soc 41: 783-784.
3. Lin JT, Arcinas A, Harden LR, Fagerquist CK (2006) Identification of (12- ricinoleoylricinoleoyl)diricinoleoylglycerol, an acylglycerol containing four acyl chains, in castor oil by LC-ESI-MS. J Agric Food Chem 54: 3498-3504.
4. Lin JT, Turner C, Liao LP, McKeon TA (2003) Identification and quantification of the molecular species of acylglycerols in castor oil by HPLC using ELSD. J Liq Chromatogr Relat Technol 26: 773-780.
5. Lin JT, Chen GQ (2012) Identification of minor acylglycerols less polar than triricinolein in castor oil by mass spectrometry. J Am Oil Chem Soc 89:1773-1784
6. Lin JT, Arcinas A, Harden LA (2009) Identification of acylglycerols containing dihydroxy fatty acids in castor oil by mass spectrometry. Lipids 44, 359-365
7. Lin JT, Chen GQ (2010) Acylglycerols containing trihydroxy fatty acids in castor oil and the regiospecific quantification of triacylglycerols. J Am Oil Chem Soc 87: 1371-1379
8. Lin JT, Chen GQ (2011) Identification of diacylglycerol and triacylglycerol containing 11,12,13-trihydroxy-9,14-octadecadienoic acid in castor oil. N Biotechnol 28: 203-208
9. Lin JT, Chen GQ, Hou CT (2013) Mass spectrometry of the lithium adducts of diacylglycerols containing hydroxy FA in castor oil and two normal FA. J Am Oil Chem Soc 90: 33-38
10. Zhang H, Olson DJH, Van D, Purves RW, Smith MA (2012) Rapid identification of triacylglycerol-estolides in plant and fungal oils. Ind Crops Prod 37: 186-194
11. Lin JT, Chen GQ (2013) Identification of TAG and DAG and their FA constituents in lesquerella (Physaria fendleri) oil by HPLC and MS. J Am Oil Chem Soc 90: 1819-1829
12. Lin JT, Chen GQ (2013) Identification of tetraacylglycerols in lesquerella oil by electrospray ionization mass spectrometry of the lithium adducts. J Am Oil Chem Soc 90:1831-1836
13. Lin JT, Chen GQ (2014) Quantification of the molecular species of TAG and DAG in Lesquerella (Physaria fendleri) Oil by HPLC and MS. J Am Oil Chem Soc 91:1417-1424
14. Hayes DG, Kleiman R, Philips BS (1995) The triglyceride composition, structure, and presence of estolides in the oils of Lesquerella fendleri and related species. J Am Oil Chem Soc 73: 267-269.
15. Bhuiyan M, Tucker D, Watson K (2013) Determination and differentiation of triacylglycerol molecular species in Antarctic and non-Antarctic yeasts by atmospheric pressure-chemical ionization-mass spectrometry. J Microbiol Methods 94: 249-256.
16. Uhl O, Glaser C, Demmelmair H, Koletzko B (2011) Reversed phase LC/MS/MS method for targeted quantification of glycerophospholipid molecular species in plasma. J Chromatogr B Analyt Technol Biomed Life Sci 879: 3556-3564.
17. Song H, Hsu FF, Ladenson J, Turk J (2007) Algorithm for processing raw mass spectrometric data to identify and quantitate complex lipid molecular species in mixtures by data-dependent scanning and fragment ion database searching. Am Soc Mass Spectrom18: 1848-1858.
18. Christie WW, Han X (2010) Quantification of lipid molecular species by electrospray ionization mass spectrometry. In: Lipid Analysis, 4th edn. The Oily Press, Bridgewater, Somerset, UK 365-392.