Research Article
Sarahn Wheeler1, Flavia Brancusi1, Michael Johnston2, Ernest Graham1, and Irina Burd1,3
1Gynecology and Obstetrics, John Hopkins University School of Medicine, Baltimore, MD, United States
2Department of Neurosciences, Kennedy Krieger Institute, Baltimore, MD, United States
3Gynecology, Obstetrics & Neurology, John Hopkins University School of Medicine, Baltimore, MD, United States
Corresponding author
Sarahn Wheeler, Gynecology and Obstetrics, John Hopkins University School of Medicine, Baltimore, 2608 Erwin Road, Suite 200, Durham, NC 27710, United States, Tel: 919-681-5220; fax: 919-681-7861; E-mail: wheelersarahn@gmail.com
Received Date: 18thJanuary 2016
Accepted Date: 06th April 2016
Published Date: 11th April 2016
Citation
Wheeler S, Brancusi F, Johnston M, Graham E, Burd I (2016) Peripheral Glucose Levels at Birth and Diagnosis of Periventricular White Matter Injury in Premature Neonates. Enliven: Pediatr Neonatal Biol 3(1): 003.
Copyright
@ 2016 Dr. Sarahn Wheeler. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: Pediatric studies have shown that glycemic dysregulation is associated with adverse neurologic outcomes in premature neonates. Elevated
peripheral glucose levels are often coupled with low central glucose levels, which can harm neurologic development. We hypothesized that elevated
peripheral blood glucose levels at birth would be associated with periventricular leukomalacia (PVL), a rare but severe type of white matter brain injury
that is a precursor to cerebral palsy.
Methods: We conducted a case-control study comparing premature infants diagnosed with PVL to age-matched controls with normal cranial
ultrasounds. Peripheral glucose levels were obtained via heel sticks prior to any postnatal intervention. Standard statistics including T-tests, chi square
and logistic regression were performed using STATA version 11 (Chicago, IL).
Results: Forty-seven infants were identified, including 16 with ultrasound evidence of PVL and 31 age-matched controls. The neonates with PVL had
a mean gestational age (GA) of 27.99 ± 3.66 (mean ± SD, n = 16), average birth weight of 1121.25±607.7 grams, average cord pH of 7.24 ±0.18 and
average birth glucose of 80.43 ± 26.6 mg/dL. The age-matched controls had a mean GA of 27.83 ± 3.46, mean birth weight of 1105.32 ± 477.3, average
cord pH of 7.26 ± 0.14, and average birth glucose of 72.35 ±16.3 mg/dL. The difference between blood glucose levels between PVL and control groups
was not statistically significant. However, logistic regression modeling revealed an odds ratio of 0.24 (95% CI: 0.063-0.902) for abnormal ultrasound
findings associated with glucose less than 85 mg/dL. The positive predictive value was 62% and the negative predictive value was 79%.
Conclusion: Peripheral glucose levels at or below 85 mg/dL at birth was associated with a decreased rate of PVL in preterm infants. These findings
suggest that glycemic autoregulation might have a protective neurologic effect on premature infants. Additionally, birth heel-stick glucose levels may
prove to be a valuable risk stratification tool for preterm infants.
Keywords
Glucose; Periventricular leukomalacia; Premature
Introduction
Over 400,000 infants are born prematurely in the United States annually, resulting in excess of $26 billion in health care costs for short- and long-term disabilities [1]. One of the most devastating neurologic consequences of premature birth is periventricular leukomalacia (PVL). This condition affects up to 10% of neonates born prior to 33 weeks and is known to lead to cerebral palsy [2]. It is critically important to understand as early as possible which neonates are at risk of PVL and associated adverse neurological outcomes, and tailor care accordingly.
PVL is a white-matter brain injury that is most often associated with ischemia in the periventricular areas. PVL is diagnosed based on ultrasound or MRI demonstration of characteristic lesions caused by multiple discrete foci of necrosis and coagulation near the lateral ventricles that resorb to form gliosis scars or cysts. These scarred areas are surrounded by damaged pro-oligodendricytes, edema, and loss of capillaries. The premature brain is more likely to have underdeveloped vasculature in the deep and subcortical areas near the lateral ventricles, leaving these areas susceptible to ischemic injury leading to PVL [3].
Multiple studies in the pediatric literature suggest poor glycemic control is associated with adverse neonatal neurologic outcomes [2,4-6]. Further, there is mounting evidence that glucose dysregulation noted in the early hours of life is linked to later neurologic outcomes [5]. To gain insight into the impact of neonatal glycemic dysregulation on later neurologic development, we examined glucose levels obtained from heel sticks at birth, prior to any postnatal interventions, as a predictor of neurologic outcomes. We hypothesized that elevated peripheral blood glucose levels obtained at birth would be predictive of PVL in preterm infants.
Methods
We conducted a case-control study comparing premature infants diagnosed with periventricular leukomalacia (PVL) on cranial ultrasound at six weeks of life to age-matched controls with normal cranial ultrasounds. The study was approved by the Johns Hopkins Institutional Review Board.
Cases and controls were identified from a database of over 300 infants admitted to the neonatal intensive care unit (NICU) from April 2009 until April 2012 at a large urban academic medical center. All premature infants admitted to the NICU underwent cranial ultrasounds at six weeks of life per institutional protocol. All of these ultrasounds were reviewed by board-certified radiologists trained to identify PVL. PVL was defined as white matter changes with characteristic periventricular focal necrosis.
Inclusion criteria for the study were premature birth (less than 37 weeks gestation), admission to the NICU, and cranial ultrasound. Infants born to diabetic mothers were excluded from our analysis. Sixteen infants with PVL were included in the analysis. Thirty-one controls were age-matched 2:1 from the infants with normal cranial ultrasounds identified in the database. Glucose levels were obtained via heel sticks recorded in the infant?s chart prior to any postnatal intervention. Demographics, maternal medical conditions, and prenatal complications were compared between cases and controls.
Statistical tests were performed using STATA version 11. Continuous demographic variables were compared between the two groups using student?s T-test. Categorical data was compared via Chi-square analysis. Logistic regression was performed to determine clinically significant cut-offs for glucose values associated with poor outcomes. The positive predictive value and negative predictive value of these cut-offs were also calculated.
Results
Demographic Data by Groups
Sixteen preterm infants with ultrasound evidence of PVL were identified from our database of 300 infants admitted to the NICU from April 2009 until April 2012. Thirty-one age-matched controls that were admitted to the same NICU during the same time period and found to have normal ultrasounds at six weeks of life were also identified.
We found no statistically significant differences between the PVL and control groups in maternal demographic features including maternal age, parity, and race. The average maternal age in the PVL group was 28.25 (SD 7.22) compared to 28.64 (SD 7.68) (p=0.86). Parity ranged from zero to six in the PVL group and from zero to four in the control group (p=0.83). Both groups were majority black followed by whites; there were a total of only two Asians and one Hispanic in the entire cohort. The ethnic breakdown was not significantly different between the PVL and control groups (p=0.77) (Table 1).
|
Characteristic |
PVL group (n=16) |
Control group (n=31) |
P-value |
|
Maternal age |
28.25 (SD 7.22) |
28.64 (SD 7.68) |
0.86 |
|
Parity |
0 (0-6) |
0 (0-4) |
0.83 |
|
Race/ethnicity |
|
|
0.77 |
|
White |
5 (31%) |
7 (23%) |
|
|
Black |
10 (63%) |
22 (71%) |
|
|
Hispanic |
0 (0%) |
1 (2%) |
|
|
Asian |
1 (6%) |
1 (2%) |
|
|
Gestational age |
27.66 (SD 3.66) |
27.83 (SD 3.46) |
0.87 |
|
Infant sex |
|
|
0.62 |
|
Male |
10 (63%) |
17 (55%) |
|
|
Female |
6 (37%) |
14 (45%) |
|
|
Delivery |
|
|
0.77 |
|
Vaginal |
6 (37%) |
13 (42%) |
|
|
C-section |
10 (63%) |
18 (58%) |
|
|
Birth weight (g) |
1121.25 (SD 607.7) |
1105.32 (SD 447.3) |
0.92 |
|
NRFHT |
5 (31%) |
3 (9.7%) |
0.06 |
|
Histological chorioamnionitis |
3 (22%) |
14 (47%) |
0.12 |
|
WBC (cells/mcL) |
10,594.38 |
12,632.58 |
0.48 |
|
Steroids |
9 (56%) |
24 (77%) |
0.13 |
|
Birth glucose (mg/dL) |
80.43 (SD 26.6) |
72.3 (SD 16.3) |
0.22 |
Table 1. Characteristics of study participants. There were no statistically significant differences between PVL and control groups with regards to demographics or physiological variables.
We also found no statistically significant differences between the PVL and control groups in fetal characteristics including gestational age, gender, delivery route, birth weight, non-reassuring fetal heart rate tracing (NRFHT), cord pH, white blood cell count, and antenatal steroid administration. The average gestation age in the PVL group was 27.66 weeks (SD 3.66) compared to 27.83weeks (SD 3.46) (p=0.87). Sixty-three percent were male in the PVL group, while 55% were male in the control group (p=0.62). In both groups, the majority of babies were delivered via cesarean section ? 63%in the PVL group and 58%in the control group (p=0.77). The average birth weight in the PVL group was 1121.25 grams (SD 607.7) and 1105.32 grams (SD 477.3) in the control group (p=0.92). There was a trend toward more frequent non-reassuring fetal heart rate tracing (NRFHT) in the PVL group (31%) compared to the control group (9.7%); however, this did not reach statistical significance (p=0.06). There was no statistically significant difference in cord pH between the groups. The mean cord pH in the PVL group was7.24 (SD0.18) and 7.26 (SD 0.14) in the control group (p=0.52). White blood cell count at birth was10,594 cells/mcL in the PVL group and 12,632cells/mcL in the control group (p=0.48). There was also no statistically significant difference in the rate of antenatal steroids: 56% in the PVL group and 77% in the control group (p=0.13).
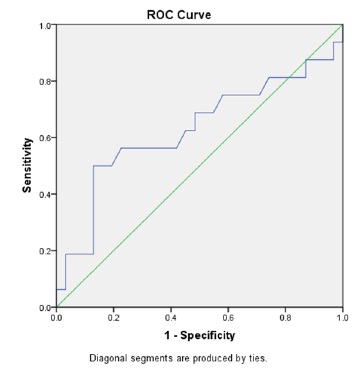
Our primary exposure was heel stick glucose level at delivery, prior to postnatal intevention. There was no statistically significant difference when comparing the mean glucose level between the PVL and control groups. The average glucose level in the PVL group was 80.43 mg/dL (SD 26.6) compared with an average of 72.3 mg/dL (SD 16.3) in the control group (p=0.22). Although there was no difference in mean glucose levels, after comparing multiple cut-points we found that infants with glucose above 85 mg/dL were statistically more likely to have PVL on their cranial ultrasound. Logistic regression revealed an odds ratio of 0.24 (95% CI 0.063-0.902). Furthermore, we found that the positive predictive value for glucose above 85 mg/dL was 62% and the negative predictive value was 79% for PVL changes on ultrasound at six weeks of life (Figure 1). An ROC curve using glucose to predict PVL was generated (Figure 2).
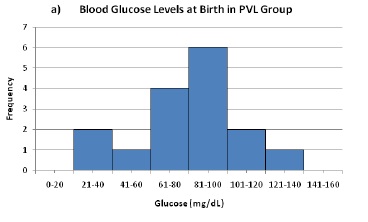
a) Blood Glucose Levels at Birth in PVL Group
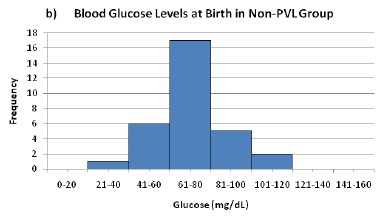
b) Blood Glucose Levels at Birth in Non-PVL Group
Figure 1. Measurements of peripheral blood glucose at birth for PVL (a) and control (b) groups. Average birth glucose was not significantly different between groups, but incidence of PVL was significantly associated with birth glucose above 85 mg/dL.
Figure 2. ROC curve using glucose level at birth to predict PVLM. AUC 0.63.
Discussion
We conducted a two-to-one age-matched case-control trial comparing 16 infants diagnosed with PVL and 31 controls. We found that blood glucose less than 85 mg/dL measured via heel stick at birth was associated with a 76% lower risk of PVL on cranial ultrasound at six weeks of life. Our study suggests a novel application of a common technique could be used as an additional risk stratification tool to identify infants most at risk for PVL and associated neurologic sequelae.
Our findings are consistent with several observations noted in the pediatric literature. It is well described that premature and low birth weight infants (below 1500g) are at increased risk for hyperglycemia [2]. There is also extensive literature linking this hyperglycemia with very poor outcomes ranging from retinopathy of prematurity, sepsis, and poor neurologic outcomes. Alexandrau et al. published a study of 143 preterm infants and found that hyperglycemia (plasma glucose over 150 mg/dL) in the first day of life was associated with an increased risk of white matter loss on MRI [6]. In contrast to the findings of Alexandrau et al., our findings point to an even lower threshold for birth glucose measurements that are linked to an elevated risk of white matter injury ? 85 mg/dL instead of 150 mg/dL. This much lower threshold for glucose levels is likely given our measurements are obtained at birth, prior to any feeding or oral glucose supplementation. Our findings are may seem in contrast to Riddle, et al. who found elevated glucose levels were neuroprotective in their sheep model of white matter injury. Our findings likely differ given the blood samples in the Riddle et al work are taken for central arterial samples. Our study utilizes samples from venous peripheral blood where higher glucose levels may represent poor autoregulation leading to higher glucose levels in the periphery when it should be shunted to the central circulation [9].
Prior studies have attributed the glycemic dysregulation-associated poor outcomes to postnatal interventions such as nutritional supplements. For example, Hays found that 60% of low birth weight neonates supplemented with parenteral feeds had episodes of glucose above 150 mg/dL, and this hyperglycemia was associated with intraventricular hemorrhage, sepsis, and death [4]. Our findings are consistent with the mounting evidence that neurologic damage from glycemic dysregulation may occur in utero. Nadeem et al. published a study examining term infants with hypoxic ischemic encephalopathy (HIE). They found glucose measurements within six hours of life were predictive of HIE, whereas glucose values obtained after that time were not predictive [5]. Additionally, postnatal glycemic control has had limited success in improving outcomes. Sinclair et al. published a Cochrane review examining interventions to prevent hyperglycemia in low birth weight infants [2]. They concluded that most of the interventions were not successful at improving major morbidities. It is possible that these postnatal interventions did not improve major morbidity because much of the neurologic damage associated with poor glycemic control occurred before birth.
The mechanism by which glycemic dysregulation damages the preterm brain is unclear. It is possible that elevated glucose levels are toxic to the developing neural tissues. This phenomenon is seen in other disease processes such as diabetes, where exposure to high glucose levels leads to nerve damage. However, it is also possible that peripheral hyperglycemia is noted because these premature fetuses are unable to auto-regulate and shunt glucose to the central system. Even though peripheral hyperglycemia is noted, this might be indicative of central hypoglycemia. It is well known that neural tissue is dependent upon glucose for energy; therefore, low glucose supply would likely lead to suboptimal neural functionality.
We also note a trend toward increased incidence of non-reassuring fetal heart tracing in the fetuses later diagnosed with PVL (31% in the PVL group vs. 9.7% in the control group, p=0.06). This observation is consistent with previous findings linking observations on fetal monitoring with later diagnosis of PVL [7].
Our findings must be considered along with several limitations. Because PVL is a rare event, our study is limited by small sample size. Our study was also conducted at a single urban academic medical institution limiting the generalizability of our results. There may be hematologic differences between capillary blood (collected via heel stick) and venous blood, so we caution that our results are not generalizable to venous blood samples.
Our study is strengthened by its novel data and expert radiology staff who read the ultrasounds. Although MRI is commonly believed to be more sensitive to detect PVL, we chose conduct our study utilizing ultrasound, which is less expensive and more readily available, to add to the generalizability of our results. Additionally, there is data to suggest that ultrasonography successfully detects cystic white matter injuries such as PVL at the same rate as MRI [8]. We chose to focus on PVL, a relatively rare event, because of its severe neurological consequences and ability to be diagnosed with high confidence shortly after birth, as opposed to other adverse neurological outcomes associated with prematurity that manifest later in life.
We have shown that the same correlation between higher glucose levels and poor outcomes observed postnatally exists at birth and therefore likely in utero. The clinical implications of this research have the potential to be far-reaching. Peripheral birth glucose may prove to be a valuable, inexpensive, readily-available risk stratification tool for preterm infants. Although rates of survival for preterm infants surviving into childhood and beyond are high, very few interventions have improved their neurologic outcomes. The early identification of white matter brain injuries is important for providing patient counseling and individualized treatment and rehabilitation plans. Furthermore, PVL is a known antecedent to cerebral palsy, and early detection of at-risk infants is critical to improving care and outcomes.
Declaration of Interest
The authors report no declarations of interest
References
- Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Kirmeyer S, et al. (2011) Births: final data for 2009. Natl Vital Stat Rep 60: 1-70.
- Sinclair JC, Bottino M, Cowett RM (2011) Interventions for prevention of neonatal hyperglycemia in very low birth weight infants. Cochrane Database Syst Rev CD007615.
- Rorke LB (1992) Anatomical features of the developing brain implicated in pathogenesis of hypoxic-ischemic injury. Brain Pathol 2: 211-221.
- Hays SP, Smith EO, Sunehag AL (2006) Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics 118: 1811-1818.
- Nadeem M, Murray DM, Boylan GB, Dempsey EM, Ryan CA (2011) Early blood glucose profile and neurodevelopmental outcome at two years in neonatal hypoxic-ischaemic encephalopathy. BMC pediatrics 11:10.
- Alexandrou G, Skiold B, Karlen J, Tessma MK, Norman M, et al. (2010) Early hyperglycemia is a risk factor for death and white matter reduction in preterm infants. Pediatrics 125: e584-e591.
- Okamura M, Itakura A, Kurauchi O, Hayakawa F, Mizutani S, et al. (1997) Fetal heart rate patterns associated with periventricular leukomalacia. Int J Gynaecol Obstet 56: 13-18.
- Miller SP, Cozzio CC, Goldstein RB, Ferriero DM, Partridge JC, et al. (2003) Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol 24: 1661-1669.