Authors: Pravin Ubale, MD1*, Rameshwar Mhamane, MD2, Sneha Raju3, Pallavi Gaur3, Pankaj Sharma3, and Rushikesh Nalavade3
1 Associate Professor, Department of Anaesthesiology, TNMC &BYL Nair Hospital, Mumbai
2 Assistant Professor, Department of Anaesthesiology, TNMC & BYL Nair Hospital, Mumbai
3 Resident, Department of Anaesthesiology, TNMC & BYL Nair Charitable Hospital, Mumbai
Corresponding author
Dr. Pravin Ubale, Associate Professor, Anand Bhavan, B. Building, Flat no.16, 4th floor, TNMC and BYL Nair Charitable Hospital, Bombay Central, Mumbai- 400008, Maharashtra, India,
Tel: 9322211472;
E-mail: drpravinubale@gmail.com;
Received Date: 04 July 2015;
Accepted Date: 10 July 2015;
Published Date: 14 July 2015
Citation
Ubale P, Mhamane R, Raju S, Pallavi G, Sharma P, et al. (2015) Anaesthetic Management of a Child with Laryngeal Papillomas. Enliven: J Anesthesiol Crit Care Med 2(7): 019.
Copyright
@ 2015 Dr. Pravin Ubale. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The objective of this work is the docking of incensole isolated from Boswellia carterii. The molecular target is human aldose reductase (ALR2) whose crystallographic structure was obtained from protein data bank with access code 2NVC. Docking analysis was performed using the MOE, 10.2009. The docking studies of the ligand incensole with ALR2 showed that this is a good inhibitor molecule. Therefore, incensole can be considered for developing into a potent ALR2- inhibitor drug.
Keywords
Incensole; Boswellia carterii; Human Aldose Reductase; Diabetes
Introduction
Incensole was first isolated as a new macrocyclic diterpene cembrane alcohole from frankincense (Boswellia carterii Birdw) by Corsano and Nicoletti [1]. It has several pharmacological activities such as anti-inflammatory and neuroprotective [2], antiproliferative effect [3], cytotoxic activity [4], and its derivative incensole acetate has antidepressive-like action [5]. At the beginning of 2012 there was a patent work [6] has investigated the use of boswellic acids for prophylaxis and treatment the inflammation of islets of Langerhans. The mixture used for this study was combination of acetyl-11-keto-β-boswellic acid, 11-keto-β-boswellic acid, β-boswellic acid, acetyl-β-boswellic acid, 9,11-dehydro-β-boswellic acid, acetyl-9,11-dehydro-β-boswellic acid, α-boswellic acid, acetyl-α-boswellic acid, 11-dehydro-α-boswellic acid, acetyl-9,11-dehydro-α-boswellic acid, lupeolic acid, acetyl lupeolic acid, 12-ursene-2-diketone, incensole and incensole acetate. Diabetes mellitus (DM) is one of the widely spread chronic diseases. It is characterized by a group of disorders such as disturbances in carbohydrates, proteins and fat metabolism caused by defective of insulin secretion, insulin action or both [7,8]. Nowadays, available oral hypoglycemic drugs showed characteristic profiles of side effects. Therefore, the development of new antidiabetic drug with minimal side effects is still great challenges to researchers. In such cases they must oriented to naturally derived drugs with less degree of hazards. Enzyme aldose reductase plays essential role in polyol pathway, it catalyzes the conversion of glucose to sorbitol, and then sorbitol dehydrogenase converts sorbitol to fructose. This enzyme has a low affinity for glucose, so that it has little catalytic activity for the conversion of glucose to sorbitol. However, in diabetes mellitus, the polyol pathway accelerates the formation of sorbitol in insulin-insensitive tissues. So, aldose reducatse inhibitors will be able to prevent the reduction of glucose to sorbitol and reducing the complications associated with diabetes [9,10]. Recently, bioinformatics become very important tools to determine and estimate the targets for different ligands. We have used bioinformatics tools to estimate to what degree incensole is a suitable ligand to human aldose reductase (ALR2) that has evolved as a promising therapeutic target for the treatment of diabetic long-term complications. The X-ray crystallographic structures of the target receptors of human aldose reductase (PDB code: 2NVC) was obtained from RCSB Protein Data Bank website, http://www.rcsb.org/pdb. In silico docking was performed using MOE 2009.10 package software.
Experimental
Plant Material
The oleogum resin of Boswellia carterii Birdw (Bursearceae) was purchased from the local herbal stores in Mansoura on May 2012. It was authenticated by comparison with a genuine sample kept in the Drug Museum of Pharmacognosy Department, Faculty of Pharmacy, Cairo University. Further confirmation is by reviewing literature concerning the phytochemical differentiation between Boswellia species [11]. A voucher specimen has been deposited in the herbarium of our department under the number (B. carteri/OGR/105).
Isolation of Incensole
A weight of 100 gm olibanum resin was purchased from commercial source and powdered in electric mixer. The powder was extracted with distilled methanol. The collected extract was dried under reduced pressure to give 20 g total extract. The total olibanum extract was applied on to the top of a silica gel column eluted with pet. ether containing increased proportions of EtOAc till 10% EtOAc. Fractions were monitored by TLC and using freshly prepared anisaldehyde as a dyeing reagent and heated until coloured spots appeared then left at room temperature for approximately 15 -30 min. for colour development and significant spots could be visually detected. Fractions 7- 13, eluted with pet. ether: EtOAc (9.8 : 0.2), revealed the presence of one brown major spot with Rf = 0.37 using pet. ether: EtOAc (9 : 1) as developer. 1H-NMR, 13C-NMR and MS data were used to identify the isolated incensole in addition to comparison with published data [12].
Molecular Docking
By reviewing literature [8,13], it was found that several targets were used for simulation of anti-diabetic drugs. The X-ray crystallographic structure of the target receptor human aldose reductase which is crystallized together naphtho[1,2- d]isothiazole acetic acid derivative with resolution 1.65 Å obtained from online database Protein Data Bank (http://www.rcsb.org/pdb) with access code: 2NVC. Molecular modeling studies were carried out on personal laptop with Pentium® Dual-Core CPU, 2.30 GHz processor, 4.00GB memory with Windows 7 home premium operating system using Molecular Operating Environment (MOE, 10.2009) software. All required minimizations were performed with MOE until a RMSD gradient of 0.05 kcal mol-1Å-1 with MMFF94X force field and the partial charges were automatically calculated. The enzyme was prepared for docking studies where: (i) all co-crystallized water molecules were removed. (ii) Ligand molecule was removed from the enzyme active site. (iii) Hydrogen atoms were added to the structure with their standard geometry. (iv) MOE Alpha Site Finder was used for the active site search in the enzyme structure and dummy atoms were created from the obtained alpha spheres. (v) The obtained model was then used in predicting the ligand enzyme interactions at the active site. (vi) London dG scoring function is used as default setting to estimate the free energy of binding of the ligand from a given pose.
Results and Discussion
Identification of Incensole
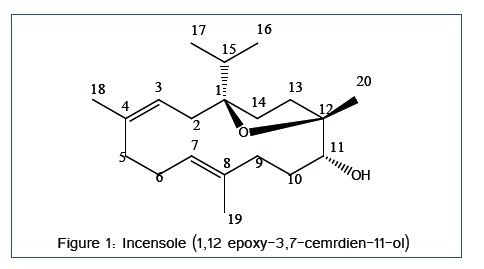
Incensole was isolated as pale yellow oil; soluble in methylene chloride and ethyl acetate. It is diterpene cembrane skeleton. EI-MS: molecular ion peak at m/z 306 (C20H34O2). 13C-NMR (100 MHZ; CDCl3) showed twenty carbon signals at δ : 88.7 (C1), 42.4 (C2), 121.8 (C3), 134.7 (C4), 38.8 (C5), 24.9 (C6), 125.2 (C7), 134.3 (C8), 33.7 (C9), 30.8 (C10), 75.6 (C11), 84.2 (C12), 36.4 (C13), 30.6 (C14), 34.8 (C15), 18.1 (C16), 18.2 (C17), 16.2 (18), 18.3 (19), 20.8 (20). 1H-NMR (400 MHZ; CDCl3) showed the following signals at δ: 5.08 (H3), 2.31 (H6, t, J=1.83), 5.06 (H7), 3.27 (H11, d, J= 9.64), 0.87 (H16), 0.89 (H17), 1.47 (H18), 1.60 (19), 1.04 (20). The physical and spectral data of the isolated compound is entirely consistent with published data of incensole [12] (Figure 1).
Results of Docking Study
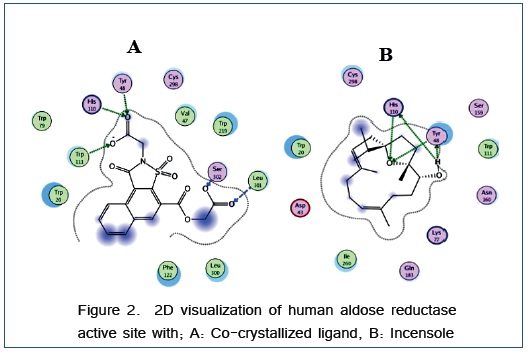
Docking protocol was verified by re-docking of the co-crystallized ligand in the vicinity of the active site of the enzyme with energy score (S) = − 16.1342 Kcal/ mol and root mean standard deviation (RMSD) = 1.7772 (Figure 2). The co-crystallized ligand interacts with five of conserved amino acids namely; Tyr48, His110, Trp111, Ser302 and Leu301. This interaction appears in hydrogen bonds (their score and interatomic distances were cited in table 1). The isolated compound, incensole interacts with the active site of human aldose reductase by four interactions with two conserved amino acids: (i) Tyr 48 with hydrogen bonds interatomic distances 1.91 and 1.51 Å and hydrogen bonding scores are 42 and 69 %, respectively. (ii) His 110 with hydrogen bonds interatomic distances 2.77 and 2.47 Å and hydrogen bonding scores are 16 and 11 %, respectively. The number of conformations generated by incensole was sixteen which indicated its flexibility which is an important parameter to dock deeply into binding pocket. In addition, incensole fits into the active site of target enzyme (PDB: 2NVC) with good energy score (S) -12.7234 kcal/ mol and RMSD = 1.5101, suggesting its activity as aldose reductase inhibitor. Energy scores (S) and amino acid interactions for incensole and co-ligand were listed in (Table 1).
| Compounds | S kcal/ mol | Amino acid interactions | H-bond length Å | H-bonding scores % |
|---|---|---|---|---|
| Incensole | -12.7234 | Tyr 48, His 110 | 1.91, 2.77, 1.51, 2.47 | 42, 16, 69, 11 |
| Co-lig | -16.1342 | Tyr 48, His 110, Trp 111, Ser 302, Leu 301 | 1.28, 1.64, 1.56, 1.30, 1.66, 1.40 | 23, 49, 42, 31, 49, 75 |
Table 1. Binding scores and amino acid interactions of incensole and co-ligand on the active site of human aldose reductase
Conclusion
Docking analysis revealed that incensole docks well with human aldose reductase (ALR2) and it interacts through hydrogen bonding. This interaction leads to the formation of stable ALR2-incensole complex. Thus it is a good molecule and it can be considered for developing into a potent human aldose reductase inhibitor to relief the diabetes long term complications.
References
- Corsano S, Nicoletti R (1967) Structure of incensole. Tetrahedron 23: 1977-1984.
- Moussaieff A, Shein NA, Tsenter J, Grigoriadis S, Simeonidou C, et al. (2008) Incensole acetate: a novel neuroprotective agent isolated from Boswellia carterii. J Cereb Blood Flow Metab 28: 1341-1352..
- Wang F, Li Z, Liu T, Hua H (2009) Cembrane diterpenes in olibanum. Zhongguo Zhong Yao Za Zhi 34: 2477-2480.
- Li F, Xu K, Yuan S, Yan DL, Tan J, et al. (2010) Macrocyclic diterpenes from Boswellia carterii Birdwood (Frankincense). Youji Huaxue 30: 107-111.
- Moussaieff A, Rimmerman N, Bregman T, Straiker A, Felder CC, et al. (2008) Incensole acetate, an increase component, elicits psychoactivity by activating TRPV3 channels in the brain. FASEB J 22: 3024-3034.
- Amon HPT (2012) Use of Bosellic acids for the Prophylaxis and/or Treatment of Damage to and/or Inflammation of the Islets of Langerhans. Patent No. 2012/0070497.
- American Diabetes Association (2010) Diagnosis and classi?cation of diabetes mellitus. Diabetes Care 30: S42-S46.
- Balamurugan R, Stalin A, Ignacimuthu S (2012) Molecular docking of γ-sitosterol with some targets related to diabetes. Eur J Med Chem 47: 38-43.
- Ueda H, Kuroiwa E, Tachibana Y, Kawanishi K, Ayala F, et al. (2004) Aldose reductase inhibitors from the leaves of Myrciaria dubia (H. B. & K.) McVaugh. Phytomedicine 11: 652-656.
- Kato A, Yasuko H, Goto H, Hollinshead J, Nash RJ, et al. (2009) Inhibitory effect of rhetsinine isolated from Evodia rutaecarpa on aldose reductase activity. Phytomedicine 16: 258-261.
- Paul M, Brüning G, Bergmann J, Jauch J (2012) A Thin-layer Chromatography Method for the Identification of Three Different Olibanum Resins (Boswellia serrata, Boswellia papyrifera and Boswellia carterii, respectively, Boswellia sacra). Phytochem Anal 23: 184-189.
- Alemika TE, Onawunmi GO, Olugbade TA (2004) Isolation and characterization of incensole from Boswellia dalzielii stem bark. Journal of Pharmacy and Bioresources 1: 7-11.
- Bharti SK, Krishnan S, Kumar A, Rajak KK, Murari K, et al. (2012) Antihyperglycemic activity with DPP-IV inhibition of alkaloids from seed extract of Castanospermum australe: Investigation by experimental validation and molecular docking. Phytomedicine 20: 24-31.