Original Article
Nicole Blaize*1, Erica Zartman1, Thomas Biel2, Craig Goergen2, William Van Alstine3 Ryan Cabot4, and Sean Newcomer5
1Department of Health and Kinesiology, Purdue University, West Lafayette, IN, USA
2Department of Biomedical Engineering, Purdue University, West Lafayette, IN, USA
3Department of Comparative Pathobiology, Purdue University, West Lafayette, IN, USA
4Department of Animal Science, Purdue University, West Lafayette, IN, USA
5Department of Kinesiology, California State University San Marcos, San Marcos, CA, USA
Corresponding author
Nicole Blaize, Ph.D, 206 S. Martin Jischke Dr, West Lafayette, IN 47907, Tel: (765) 496-1407; E-mail: ablaize@purdue.du
Received Date: 22ndOctober 2014
Accepted Date: 29th December 2014
Published Date: 04th January 2015
Citation
Blaize N (2015) Impact of Maternal Exercise during Pregnancy on Arterial Function and Atherosclerosis Formation in Swine Offspring Fed a High Fat Diet. Enliven: Gynecol Obestet 2(1): 001.
Copyright
@ 2014 Dr. Nicole Blaize. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
This research article examining the influence of maternal exercise during pregnancy on vascular function in the offspring adds vital knowledge to the area of exercise physiology, fetal programming, and obstetrics research. An abundant amount of research has established the role of maternal behaviors during pregnancy on offspring health. However, much of this research has examined adverse maternal behaviors, such as smoking, drinking, stress, and over and undernutrtion, and their influence on offspring health. Research has shown that these adverse maternal behaviors can influence offspring development in utero and produce offspring that are small or large for gestational age. Changes in growth in utero can lead to greater susceptibility for diseases such as obesity, diabetes, and cardiovascular disease in the offspring later in life. Therefore, the data generated from research examining adverse maternal behaviors has helped health practitioners provide knowledge to pregnant women in order to potentially prevent or decrease the offspring’s risk for developing diseases in adulthood. While considerable research has been conducted examining the role of adverse maternal behaviors, far less has studied the impact of positive maternal behaviors, such as exercise, and in the role they play in fetal programming.
This research article examining the influence of maternal exercise during pregnancy on vascular function in the offspring adds vital knowledge to the area of exercise physiology, fetal programming, and obstetrics research. An abundant amount of research has established the role of maternal behaviors during pregnancy on offspring health. However, much of this research has examined adverse maternal behaviors, such as smoking, drinking, stress, and over and undernutrtion, and their influence on offspring health. Research has shown that these adverse maternal behaviors can influence offspring development in utero and produce offspring that are small or large for gestational age. Changes in growth in utero can lead to greater susceptibility for diseases such as obesity, diabetes, and cardiovascular disease in the offspring later in life. Therefore, the data generated from research examining adverse maternal behaviors has helped health practitioners provide knowledge to pregnant women in order to potentially prevent or decrease the offspring?s risk for developing diseases in adulthood. While considerable research has been conducted examining the role of adverse maternal behaviors, far less has studied the impact of positive maternal behaviors, such as exercise, and in the role they play in fetal programming. Recent research has shown that maternal exercise during pregnancy can improve cognitive function, improve glucose handing, and improve regulation of the cardiovascular system in the offspring. Consequently, we examined the role of maternal exercise during pregnancy and its impact on vascular function and atherosclerotic disease susceptibility in 4 and 8-month old swine offspring fed a high fat diet. Currently, atherosclerotic cardio vascular disease is the leading cause of death in the United State. Exercise has been shown to positively impact adults with atherosclerosis. Furthermore, the American College of Sports Medicine (ACSM) and American College of Obstetricians and Gynecologist (ACOG) currently recommend that pregnant women exercise at a moderate intensity for 30 minutes or more on most, if not all, days of the week. Thus, we found it plausible to hypothesize that maternal exercise would positively influence the offspring?s risk for cardiovascular disease. Recently, our lab reported improved vascular function in swine offspring 48 hours after birth and decreased vascular function in swine offspring fed a standard diet at 3-, 5- and 9-months of age. To our surprise, we did not see any significant changes in vascular function or atherosclerotic lesion formation in our current study. However, it is important to note that there were no detrimental consequences of maternal exercise on the offspring?s vasculature. Despite the fact that exercise during pregnancy has many beneficial health outcomes in the mother and offspring, research still indicates that only 15% of women meet the ACOG and ACSM recommendations for exercise during pregnancy. Literature has reported that while some obstetricians follow current ACOG guidelines and routinely recommend exercise to healthy, pregnant patients, a significant percentage do not. In a study examining women?s beliefs and influences in regards to exercise during pregnancy, data suggest that women would be more likely to exercise if their obstetrician encouraged them to do so. It is possible that some obstetricians do not feel as if there is sufficient evidence to support prescribing exercise during pregnancy and consequently do not recommend it to their pregnant patients. The results of the current study could be used to provide knowledge to obstetricians about the safety of maternal exercise on long-term cardiovascular health outcomes in the offspring. With this information, obstetricians may feel more comfortable encouraging pregnant women to meet the guidelines set by the ACOG and ACSM.
Abstract
Previous research has revealed that maternal exercise during pregnancy can impact offspring?s vascular phenotype upto 9 months of age in swine. This work utilized animals that were fed a standard diet and therefore did not develop atherosclerosis. Thus, the purpose of this investigation was to test the hypothesis that maternal exercise during pregnancy will improve femoral and coronary artery vascular function and decrease atherosclerotic lesion formation at 4 and 8 months of age in swine offspring fed an atherogenic diet. Pregnant sows were divided into exercise-trained (n=8) or sedentary (n=8) groups. The exercise-trained group trotted on a treadmill 5 days/week for 25-40 minutes at 2.5mph throughout gestation. After weaning, offspring were group housed and fed a high fat diet for the duration of the study. We assessed offspring feed consumption and cage behavior at 8-months of age. Ultrasound of the carotid arteries was used to quantify in vivo cyclic strain. The left anterior descending (LAD) and femoral arteries were harvested at 4- and 8-months of age in order to assess vascular function and histology. There were no significant differences in litter characteristics between offspring from exercise-trained and sedentary mothers at either time point. Feed consumption, cage behavior, carotid artery systolic diameter, diastolic diameter, and Green-Lagrange circumferential cyclic strain were not significantly different between exercise and sedentary offspring at 8 months of age. Additionally, offspring from exercise-trained and sedentary mothers did not display significantly different atherosclerotic lesion formation or vasorelaxation responses to bradykinin or sodium nitroprusside at 4 or 8 months of age. In conclusion, maternal exercise does not significantly alter behavior or LAD and femoral artery vascular function and atherosclerotic lesion formation in adult swine offspring fed a high fat diet.
Introduction
Atherosclerotic cardiovascular disease (CVD) is currently the leading cause of death in the U.S. [1]. While it was long thought to be a disease predominantly affecting the elderly, recent research has indicated that atherosclerotic lesions can be present in infants, indicating a role for the intrauterine environment on CVD susceptibility [2]. Exercise in children and adults have been shown to have a positive impact by lowering CVD risk. However, little work has been done examining the role of exposure to exercise in utero on CVD susceptibility in offspring.
Similar to children and adults, research has found that maternal exercise during pregnancy can have many positive health benefits for the mother, including decreased risk for excessive weight gain, gestational diabetes, preeclampsia, and help with postpartum recovery [3-5]. The current guidelines set by the American College of Obstetricians and Gynecologists (ACOG) and the American College of Sports Medicine (ACSM) recommend pregnant women exercise at a moderate intensity for 30 minutes on most, if not all, days of the week [6]. While the maternal beneficial effects of exercise during pregnancy have been extensively examined, relatively little attention has been given to the influence on offspring health.
Recent research has begun to examine the role of maternal exercise during pregnancy on offspring health outcomes. Maternal exercise has been reported to improve offspring cognitive function, metabolic health, cardiac function, and decrease cancer risk [5,7-11]. In addition, our laboratory has recently reported that maternal exercise during pregnancy can alter vascular function in swine offspring up to 9 months of age [12,13]. One of the limitations of this previous work was that it only utilized healthy animals that did not develop atherosclerosis. Furthermore, these studies did not characterize coronary arteries, a vasculature region that is highly susceptible to arteriosclerosis. Based on our prior reports, it is unclear if the vascular function differences seen in thoracic and femoral arteries in healthy swine offspring would also be observed in coronary arteries due to the heterogeneity of the vessels [14-16]. Consequently, it is important for investigations to examine coronary artery vascular function and atherosclerosis development in swine offspring fed an atherogenic diet. The purpose of the current study was to test the hypothesis that maternal exercise during pregnancy would improve femoral and coronary artery vascular function and decrease atherosclerotic lesion formation at 4- and 8-months of age in swine offspring fed a high fat diet. In addition, we utilized ultrasound techniques, which are commonly used in clinical settings to monitor cardiovascular disease, to measure vessel dynamics in a subset of carotid arteries. We also examined the influence of offspring feed consumption and cage behavior as these factors have been shown to impact vascular endothelial function.
Methods
Animal Model, Exercise Training and Heart Rate Measurements
This investigation was carried out in accordance with the animal care and use protocol approved by the Purdue Animal Care and Use Committee. Sixteen 6-month-old pubertal gilts were utilized for this study. Ovarian cycles were synchronized by feeding an orally active progestin (Altrenogest, Intervet, Millsboro, DE, USA) 2 weeks prior to artificial insemination from a single boar. Gilts were then randomly assigned to two groups: exercise-trained (n=8) or sedentary (n=8). All gilts were individually housed in an environmentally controlled large animal housing facility and fed a standard gestational diet (3.25 kg/day). Exercise training consisted of treadmill exercise throughout gestation for 20-45 minutes a day, 5 days a week. Resting heart rates were measured for the exercise and sedentary gilts at weeks 1, 6, 9, and 15 of pregnancy in order to determine the effects of the exercise training protocol (Polar S810, Polar Electro Inc., Lake Success, NY, USA). In addition, the exercise-trained mothers exercising heart rates were assessed at weeks 7, 10 and 15 of pregnancy. During the exercise training, sedentary gilts were loaded into a cage adjacent to the treadmill in order to control for environmental stimuli. All gilts were weighed weekly. During the last week of gestation (week 16) the exercise group was not trained as all gilts were transferred to a different facility in order to prepare for farrowing. Normal gestational length in swine is 114 days.
Litter Characteristics and Animal Housing
Litter numbers and animal weights were recorded at birth. After weaning the offspring from each litter were randomly assigned to 4- or 8-month groups and weighed bi-monthly. For reference, offspring referred to as sedentary or exercise represents the mother being sedentary or exercise-trained during pregnancy. None of the offspring exercised throughout the course of this study. We chose to utilize primarily female offspring based on previous data showing vascular function differences in female offspring [13]. However, not all mothers gave birth to female offspring, which meant that 4 males (2 exercise and 2 sedentary) were used at each time point. All offspring were group housed and therefore males were castrated within the first 5 days of birth. Additionally, offspring were fed an atherogenic diet (diet composition: standard chow supplemented with 2% cholesterol, 4.7% hydrogenated coconut oil, 8.4% hydrogenated soy bean oil, 0.7% collate, 5% and high fructose corn syrup; Purdue feed mill, West Lafayette, IN, USA) ad libitum throughout the course of the study starting at 8 weeks of age. The percentages listed in the diet composition are representative of the amount of weight of each supplemental ingredient added to the standard chow diet.
Food Consumption and Behavior Analysis
Food consumption and behavior were assessed in the 8-month offspring within 2 weeks of sacrifice. During behavior monitoring the offspring were removed from their group pen and housed in individual pens within the same building. This allowed the animals to be able to see and hear their littermates in adjacent pens. Offspring activity was monitored as previously described using a monopod-mounted video camera and stored on a 6 channel DVR system (Nuvico, Easynet Ultra Series DVR, ED-U1600/U3200, Englewood, NJ). All offspring were observed for a 24-hour period. The percentage of time the animal spent feeding, drinking, resting, and walking was recorded and analyzed offline by instantaneous scan sampling using 10-minute intervals [17]. Resting behavior was defined as the animal sitting, lying sternally, or lying laterally. Walking was defined as the animal being in a standing position. The percentage of time spent in a behavior was estimated by adding the number of observations of each particular activity and dividing that number by the total number of observations. We assessed feed consumption by weighing the food before and after the observation period.
Non-Invasive Ultrasound
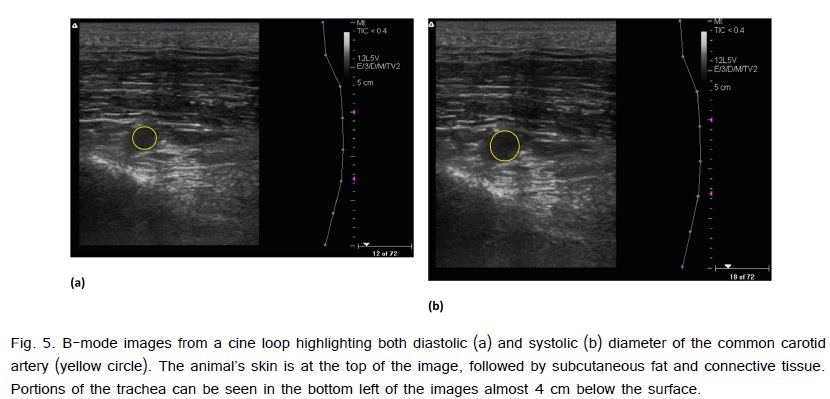
The carotid arteries of a subset of offspring from sedentary (n=3) and exercise (n=4) mothers from 6 to 8 months of age were imaged with B-mode ultrasound. During imaging, the offspring were removed from group housing and placed in a small individual pen within the same building. The pen was small enough to restrict motion, meaning the animals did not need to be anesthetized or restrained in a sling. Ultrasound data were collected while each offspring remained still and standing using a 128-element linear 38 mm transducer (model 12L5V, 5-12 MHz) and ultrasound/Doppler system (Terason T3000; Teratech, Burlington, MA). The probe was placed on the underside of each animal?s neck and positioned vertically to visual the common carotid artery. Systolic (maximum) and diastolic (minimum) diameters were quantified using ImageJ Software (NIH, Bethesda, MD, USA). Multiple measurements were made for each animal with an average of 6.9±2.1 (maximum: 17; minimum: 2).
Tissue Harvest and Storage
Offspring from the exercise-trained and sedentary groups were randomly selected to be euthanized through electrical stunning followed by exsanguination before the return of consciousness at 4 (n=16) and 8 (n=16) months of age. Left anterior descending (LAD) and femoral arteries were collected immediately after death of the animal. The left femoral and proximal portion of the LAD was stored in neutral buffered formalin for histological purposes. The right femoral and distal LAD was placed in Krebs solution for in vitro wire myography analysis.
Vascular Function Experiments
The right femoral and distal LAD were removed from Kreb?s solution, cleaned of connective tissue, and cut into two sections, each approximately 3 mm in length. A stereomicroscope (PZMIII, World Precision Instruments, Sarasota, FL, USA) in combination with ImageJ software were used to determine arterial rings axial length and outer and inner diameters. Femoral and LAD rings were then mounted in an alternating series on wire myographs (Myobath II, World Precision Instruments, Sarasota, FL, USA), placed in a 20 ml heated bath of Kreb?s bicarbonate solution (37°C), bubbled with a mixture of 95% O2 and 5% CO2, and set to 5 or 8 grams of tension for LAD and femoral arteries, respectively. These tensions were determined to be the optimal point in the length-tension relationship based on historical data from arterial segments of similar size [18]. All rings were preconstricted using prostaglandin F2? (PGF2 ?; 30 ?M) and allowed to reach tension equilibrium. Endothelium-dependent and independent, dose-dependent vasorelaxation was then assessed using cumulative addition of bradykinin (BK; 1011-10-4 M) and sodium nitroprusside (SNP; 10-10 -10-4 M) respectively.
Histology
The proximal LAD and left femoral arteries were fixed in neutral buffered formalin. Two sections from each artery were cut, embedded in paraffin, and mounted on glass slides. Each section was then stained with haematoxyloin and eosin (H&E). Atherosclerotic lesions were then scored using the Stary scoring system by a licensed pathologist blinded to the intervention. In order to quantify the amount of atherosclerotic development in the arteries, each section was divided into 4 quadrants with each receiving a Stary score (i.e., two sections with 4 quadrants each equals 8 Stary scores per artery). The scores were then analyzed in two ways: total vessel and severity. The total vessel score was calculated by averaging the 8 Stary scores per vessel, while the severity score was calculated by averaging the 2 highest Stary scores per vessel.
Statistics
Student?s t-tests were used to compare weights and heart rates between sedentary and exercise mothers, as well as systolic diameter, diastolic diameter, and Green-Lagrange circumferential cyclic strain of carotid arteries between exercise and sedentary offspring. Litter characteristics, vessel characteristics, behavior, and dose-response curves for BK and SNP were analyzed using a repeated measures analysis of variance (ANOVA) where mother was nested in intervention. Histology data were analyzed using an unpaired, non-parametric t-test with a Mann-Whitney post-hoc analysis. Statistical significance was set at p<0.05. All data are presented as mean ± SE.
Results
Maternal and Litter Characteristics
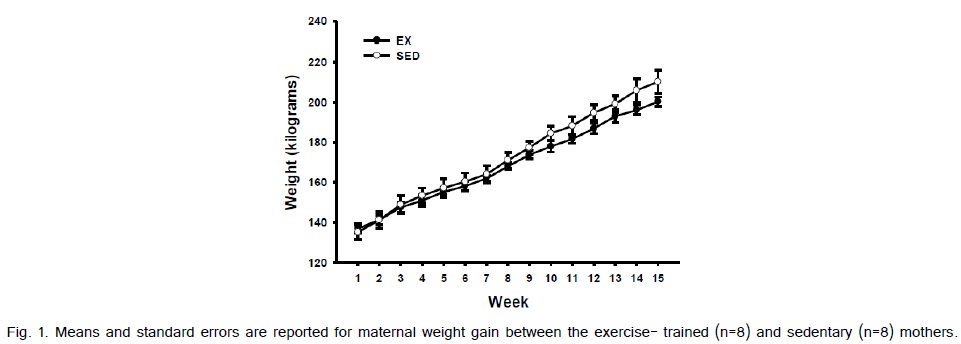
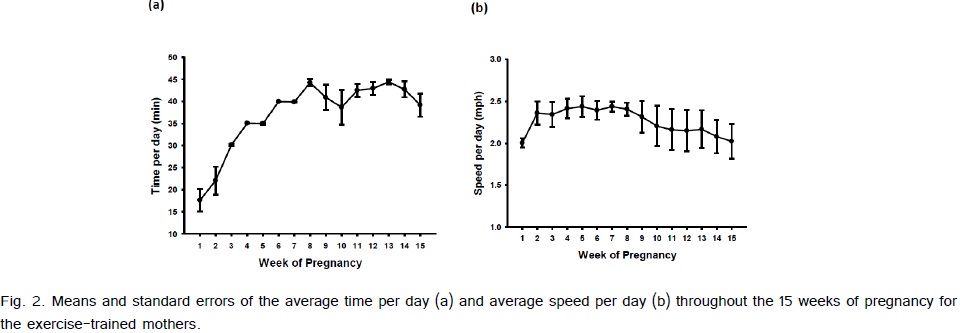
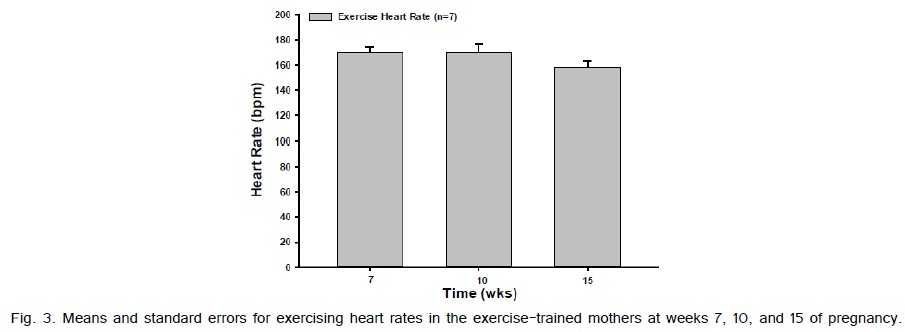
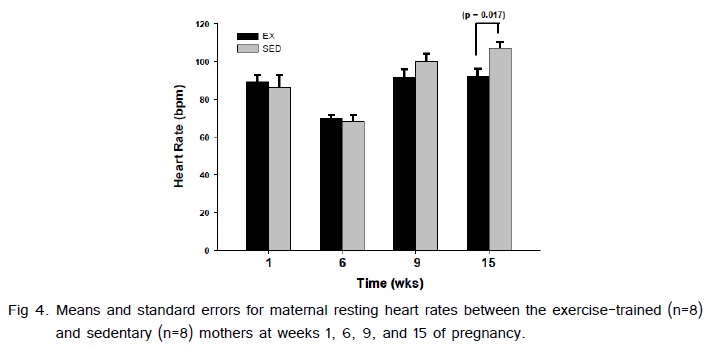
Weight gain from pre-pregnancy to the last week of pregnancy between the exercise-trained and sedentary gilts was not significantly different (Fig. 1). The average running time and speed are shown in (Fig. 2). Exercise-trained gilts were exercised in accordance to the ACOG and ACSM recommendations for humans, with exercising hearts rates ranging from 70-80% of age predicted max heart rate (Fig. 3). Exercise-trained gilts also had a significantly lower resting heart rate by week 15 of pregnancy compared to their sedentary counterparts (Fig. 4). There were no significant differences in litter characteristics (number and weight) between the offspring from exercise-trained and sedentary mothers at birth (Table 1).
| Exercise | Sedentary | |||||
| Mean | SE | Range | Mean | SE | Range | |
| Litter Size | 8.75 | 1.03 | 4.00-12.00 | 9 | 0.8 | 6.00-12.00 |
| Birth Weight (Kg) | 1.49 | 0.08 | 0.90-2.00 | 1.44 | 0.06 | 0.60-1.90 |
| 4 Month Weight (Kg) | 67.84 | 5.65 | 45.45-79.55 | 62.27 | 2.83 | 54.09-72.72 |
| 8 Month Weight (Kg) | 148.04 | 4.07 | 129.78-166.36 | 141.73 | 3.41 | 129.32-153.64 |
Table 1: Number of pups per litter, average birth, 4-, and 8- month weight for offspring from exercise and sedentary mothers. Range for litter size, birth weight, 4-, and 8- month weight. Values for average are given as mean ± SE. No significant differences were observed between exercise and sedentary offspring
Feed Consumption, Behavior and Weights
There were no significant differences in feed consumption and behavior between the offspring from exercise-trained and sedentary mothers at 8-months of age (Table 2). In addition, there were no significant differences in weights at 4- or 8-months of age between the offspring from exercise-trained and sedentary mothers (Table 1).
| Exercise | Sedentary | |||
| Mean | SE | Mean | SE | |
| Feed Consumption (Kg) | 2.23 | 0.25 | 2.38 | 0.23 |
| Feeding (%) | 2.78 | 0.53 | 2.43 | 0.56 |
| Drinking (%) | 1.82 | 0.45 | 1.82 | 0.26 |
| Inactive (%) | 79.52 | 3.5 | 81.44 | 2.07 |
| Walking (%) | 15.89 | 3.49 | 14.24 | 1.76 |
Table 2: Offspring feed consumption and percent of time spent in 24 hours in various cage behaviors between offspring from exercise and sedentary mothers at 8- months of age. No significant differences were observed between exercise and sedentary offspring
Ultrasound
There were no significant differences in systolic diameter (EX: 0.64 ± 0.01 cm; SED: 0.64 ± 0.02 cm) or diastolic diameter (EX: 0.47 ± 0.01 cm; SED: 0.49 ± 0.01cm) between exercise and sedentary offspring. In addition, there were no significant differences in Green-Lagrange circumferential cyclic strain (EX: 0.42 ± 0.04; SED: 0.37 ± 0.07) between the groups of offspring (Fig. 5).
Vascular Function In Vitro Wire Myography
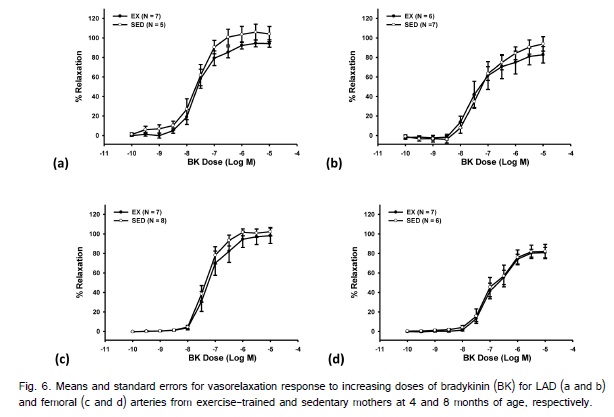
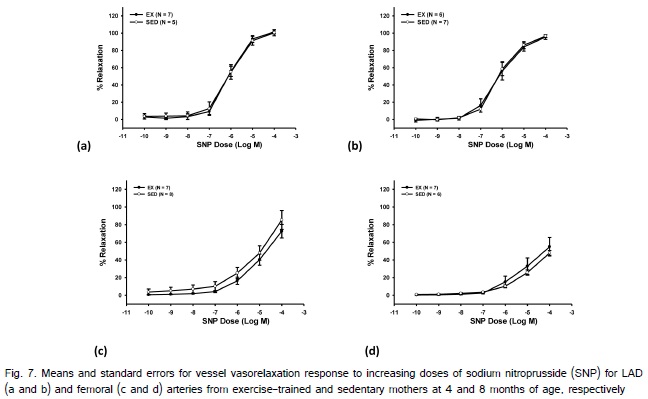
At both 4 and 8 month there were a total of 5 LAD?s (2 exercise; 3 sedentary) and 1 femoral (exercise) artery that could not be utilized due to issues with the animal condition (i.e., lesions in the myocardium) or damage to the LAD when the hearts were removed from the animal. There were no significant differences in femoral or LAD length or thickness in arterial segments used for in vitro measurements between the exercise and sedentary offspring at 4 or 8 months of age (Table 3). Exercise during pregnancy did not elicit a significant main effect for BK-induced endothelium-dependent vasorelaxation at 4- or 8-months of age in the LAD or femoral arteries (Fig. 6). SNP also did not result in a significant main effect on endothelium-independent relaxation in the LAD or femoral arteries at 4-or 8-months of age between the offspring from exercise-trained or sedentary swine (Fig. 7). There was a significant main effect of age for BK for both the LAD and femoral arteries, as well for SNP for the femoral artery with older animals displaying reduced percent relaxation.
| LAD | Femoral | |||||||
| Exercise | Sedentary | Exercise | Sedentary | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| 4 Month Length (mm) | 2.58 | 0.1 | 2.78 | 0.35 | 3.29 | 0.11 | 3.47 | 0.15 |
| 4 Month Thickness (mm) | 0.6 | 0.13 | 0.76 | 0.23 | 0.85 | 0.03 | 0.87 | 0.03 |
| 8 Month Length (mm) | 2.98 | 0.11 | 2.8 | 0.11 | 3.52 | 0.18 | 4.07 | 0.62 |
| 8 Month Thickness (mm) | 0.77 | 0.03 | 0.94 | 0.16 | 0.95 | 0.05 | 1.24 | 0.3 |
Table 3: Length and thickness of LAD and femoral arteries of offspring from exercise and sedentary mothers at 4- and 8-months of age. No significant differences were observed between exercise and sedentary offspring
Histology
There were no significant differences in average total quadrant scores for the femoral or LAD artery at 4- or 8months of age (Table 4). In addition, there were no significant differences in average severity scores for the femoral or LAD arteries at 4- or 8-months of age.
| LAD | Femoral | |||||||
| Exercise | Sedentary | Exercise | Sedentary | |||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| 4 Month Total Vessel | 0.27 | 0.06 | 0.16 | 0.36 | 0.04 | 0.04 | 0.00 | 0.00 |
| 4 Month Severity | 1.00 | 0.29 | 0.64 | 0.14 | 0.29 | 0.29 | 0.00 | 0.00 |
| 8 Month Total Vessel | 1.04 | 0.25 | 0.77 | 0.21 | 0.14 | 0.09 | 0.03 | 0.03 |
| 8 Month Severity | 1.71 | 0.31 | 1.31 | 0.27 | 0.38 | 0.31 | 0.13 | 0.13 |
Table 4: Total average and severe Stary score for LAD and femoral arteries of offspring from exercise and sedentary mothers at 4- and 8-months of age. No significant differences were observed between exercise and sedentary offspring
Discussion
It is well established that the intrauterine environment plays an important role in the offspring?s susceptibility to diseases later in life [19]. A plethora of data has described an association between negative maternal behaviors, such as smoking and poor nutrition during pregnancy, and detrimental health outcomes in the offspring. Recently, studies have reported a link between positive maternal behaviors, such as exercise, and improved offspring health outcomes [8,11,13]. Therefore, we hypothesized that exercise during pregnancy would improve femoral and coronary artery vascular function and decrease atherosclerotic lesion formation in offspring from exercise-trained compared to sedentary mothers. Furthermore, we hypothesized that these improvements in cardiovascular health outcomes would be linked to differences in offspring feed consumption and cage behavior, as well as carotid artery vessel dynamics. Contrary to our hypothesis, no significant differences between offspring from exercise?trained and sedentary mothers were observed. Therefore, the current results indicate that exercise during pregnancy does not produce an anti-atherogenic phenotype in the LAD and femoral arteries of high fat fed swine offspring up to 8?months of age.
Research has established that maternal exercise during pregnancy has many beneficial health outcomes for the mother. Maternal exercise has been shown to improve maternal fitness, decrease excessive weight gain, decrease risk for gestational diabetes and preeclampsia, and help with postpartum recovery. Consequently, guidelines set by the ACOG and ACSM recommend pregnant women participate in moderate intensity physical activity for 30 minutes on most, if not all, days during pregnancy [6]. The sows in the current investigation exercised at a similar intensity and duration that are currently recommended by the aforementioned organizations for pregnant humans. In response to the exercise protocol, the exercise-trained sows had a significant cardiovascular training adaptation as demonstrated by the lower resting heart rate, which is consistent with what has been reported in women participating in aerobic exercise during pregnancy [20].
Although research has established the beneficial health outcomes for mothers who exercise during pregnancy, only 15% of women adhere to these recommendations [3]. This disconnect could be attributed to a lack of knowledge of health consequences for their offspring. In recent years there has been growing evidence that maternal exercise during pregnancy can influence offspring cardiovascular health outcomes. Reports have demonstrated that offspring from mothers who exercised during pregnancy display lower resting heart rates, improved heart rate variability, and a trend for decreased intima-media thickness, indicating improved cardiac health in these offspring [2,11]. Our lab has previously examined vascular endothelial and smooth muscle function in standard diet fed swine offspring in response to maternal exercise during pregnancy [12-13]. Data from these studies have indicated both improved endothelial function in thoracic aortas 48 hours after birth in swine offspring from exercise-trained mothers and no differences in femoral artery endothelial function in adult swine offspring from exercise-trained or sedentary mothers. Additionally, we reported a decrease in smooth muscle function in 3, 5, and 9-month swine offspring from exercise-trained mothers fed a standard diet. Interestingly, the current results did not fall in line with our previously reported vascular smooth muscle function data in swine at 3, 5 and 9-months of age but were consistent with endothelial function data collected in the femoral arteries. Despite the lack of differences detected in the current study, we did observe a non-significant trend for reduced SNP induced relaxation in femoral arteries of offspring from exercise mothers that is consistent with what was reported previously [12]. Previous data indicated that smooth muscle function differences were greatest at 3-months of age and then decreased with increasing age. While our data are not significantly different, we did notice that the femoral smooth muscle data has a greater difference between the exercise and sedentary offspring at 4-months compared to 8-months of age (Fig. 7c and 7d). It is important to note that a similar trend for reduced vascular smooth muscle function was not apparent in LAD?s of offspring of exercised mothers, which may be a result of well-established heterogeneity between vasculatures [14-16,21].
While endothelial function testing is an important biomarker for vascular disease, it is important to consider the stage of atherosclerosis in the vessel [14]. Therefore, the LAD and femoral arteries from the high fat fed swine in the current study were histologically analyzed using the Stary scoring system for atherosclerosis. The results revealed that the LAD and femoral arteries from the offspring at 4 and 8 months had a range of scores from 0 to 3. A Stary score of 0 indicates no atherosclerotic lesions, while scores ranging from 1-3 are classified as early/minimal [22]. While the histological data confirms that the high-fat diet did produce the early stages of atherosclerosis in the animals, there were no significant differences in atherosclerotic lesion formation between offspring from exercise and sedentary mothers. The lack of vascular function, histological, and vessel stiffness differences indicates that maternal exercise during pregnancy does not produce a protective effect on the cardiovascular system in swine offspring fed a high fat diet at these time points.
In addition to determining the impact of maternal exercise during pregnancy on offspring femoral and LAD vascular function and atherosclerotic lesion formation, we also collected feed consumption and cage behavior data. Similar to the data previously discussed, we did not find any significant differences in these data between exercise and sedentary offspring. Therefore, we believe these additional measurements support the findings that exercise during pregnancy does not produce an anti-atherogenic phenotype in adult swine offspring. In addition, the methodology utilized to collect carotid ultrasound data is novel since we did not restrain or anesthetize the animal. Instead, we trained the animals to stand still in a small pen using positive reinforcement. These data show that in vivo vascular images from swine can be collected without the use of restraints or anesthesia.
The observation that the offspring were only in the beginning stages of atherosclerosis is a limitation to this study. Endothelial dysfunction occurs in response to vascular risk factors and is an early event in atherosclerosis [14]. Typically changes in endothelial function will precede structural atherosclerosis. Unfortunately, the costs of keeping swine on a high fat diet long enough to develop advanced atherosclerosis was prohibitive and not possible during this current study. However, future work examining more advanced stages of the disease in offspring from exercise-trained mothers is warranted. Another limitation to this study involved the methodology for collecting ultrasound data. While not using a restraint or anesthesia is a novel form of collecting ultrasound data on swine, there were some difficulties with our ultrasound technique as small amounts of animal movement (such as swallowing, jumping, or head movement), as well as large amounts of adipose tissue led to difficulty collecting useable images of carotid artery pulsation. In addition, the small sample size for our ultrasound data is problematic. However, due to the difficulties listed above we were unable to obtain measurements on a large number of the offspring. To our knowledge, this is the first study to collect ultrasound data on un-anesthetized swine. While the method of data collection was new, our diameter measurements and Green-Lagrange circumferential cyclic strain calculations from the ultrasound data were similar to previous reports [23-26]. Therefore, this data is a good starting point for future work to expand upon.
In conclusion, the results of this investigation demonstrate that maternal exercises during pregnancy not alter litter characteristics, behavior, or vascular disease progression in swine offspring fed a high fat diet. While we did not see the anti-atherogenic phenotype that we hypothesized, we believe it is imperative to note that we also did not see a pro-atherogenic phenotype in response to maternal exercise during pregnancy. These findings suggest that women can exercise during pregnancy without fear of harmful cardiovascular consequences to their offspring and should be used by physicians to encourage their patients to incorporate exercise into their prenatal care.
Acknowledgements
The authors thank Martin Bahls, Emily Bresin, PardisTaheripour, Celina Cheng, Spencer Harstead, Jessica Rosa, Meganne Rueter, Leigha Thompson, and Courtney Yaggi for their help with pig running. We also thank Jessica Engen for her help with sacrifices. We thank Jeremy Marchant-Forde and Rita Lockridge for their assistance with behavior analysis. Lastly, we would like to thank Amy Bogucki, Frederick Damen, Hillary Schroeder, and AlexaYrineo for their help with ultrasound data collection.
Funding
This study was supported by the American Heart Association grant #12SDG85400001 (to S.C.N.).
References
5. Clapp JF 3rd (2000) Exercise during pregnancy. A clinical update. Clin Sports Med 19: 273-286.