Authors:
Ibeh Nnanna Isaiah* Bmls, Amls, Msc, Mnaas, Mght, and Omorodion Nosa Terry Bmls, Amlsc Msc
* Health Services Department, University of Benin, Nigeria
Corresponding author
Ibeh Nnanna Isaiahm, Health services department, University of Benin, PMB 1154, Benin City, Nigeria, E-mail: andreibeh@gmail.com
Received Date: 11th August 2017; Accepted Date: 04th October 2017; Published Date: 10th October 2017
Citation
Isaiah IN,Terry ON(2017) Hibiscus flower extract (zobo)’ as a moprhological counter stain for sperm cell analysis; adult male wister rats and rabbit as a model of study Enliven: Microb Microbial Tech 4(1): 001.
Copyright
@ 2017 Ibeh Nnanna Isaiahm. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Over the years improvement has been made on the staining techniques for the morphological analysis of sperm cells ranging from the normal eosin and nigrosine combination to other more simple or complex single staining technique.
Aim: Analysis of spermatozoa morphology using hibiscus flower extract as counter stain for abnormalities and viability studies towards future applications in assisted reproductive science.
Method: 22 wister rats and rabbits sperm cells where stained to analyze their morphology, using both the conventional technique and the hibiscus flower extract to determine the reproducibility of this improved staining technique.
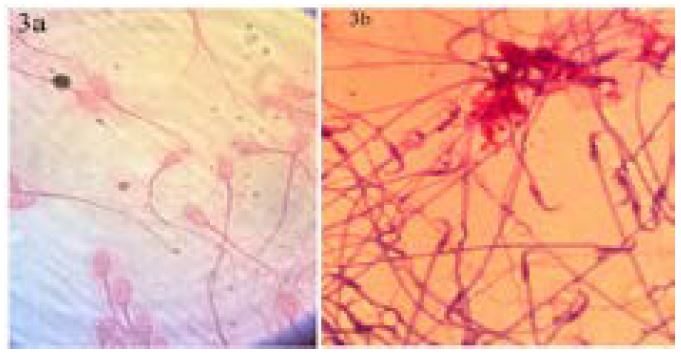
Results: From the staining technique the morphology of the sperm cells where properly noticeable from the head, body and tail which is represented in the figures 1-4.
Conclusion: In a third world country this hibiscus plants are accessible stains for quick and accurate morphological observation of wister rats and rabbit spermatozoa as an intervention tool in absence of complex staining techniques and since it is natural may have little or no effect on sperm cells.
Introduction
The morphology of spermatozoa is an tool important for a successful fertilization and early embryonic development in assisted reproductive techniques [1]. Final tiger berg classification criteria described by kruger in 1986 and the who classification are the most important spermatozoa morphological classifications. According to kruger's sperm [2], the head, neck and tail sections should be normal, the boundaries of the head should be smooth and oval and 70% of head should be coated by acrosome. The head of the sperm head length should be 4-5 m and must have a width of 2.5-3.5m. The length of the tail should be an average of 45m. The middle part must be thin and long and width should be less than 1mm, length should be of 1.5 times the length of the head. The tail should be thinner than middle piece, should be flat and not wrinkled, and should not contain broken parts and cytoplasmic debris. In addition, the sperm should not have the neck, middle piece, tail abnormalities and alargecy to plasmic droplet more than half of the spermatozoon head in the neck area [3].
Morphological features of spermatozoa are expressed in numerical values. Methods used in the staining may cause as light change in the measurement values of spermatozoa because fixatives can cause cells to shrink a little. For example, 3-5 m length and 2-3 m wide measurements are considered normal for the head of spermatozoa in the method of papanicolaou. These values change 5-6 and 2.5m-3.5m in the diff- quick staining method. In both the middle piece should be 1m thick, the length of this value should be upto1.5 times of this value. The tail section should be 45m length tapering gradually [4]. Because of these differences, we examined morphology of spermatozoa with different staining methods and aimed to find the better staining methods for laboratories in our study.
Materials and Methods
In this study randomized 22 wister rats and 22 rabbit’s semen samples were obtained from the wister rats the sperm cells where harvested from the vas deferens from the rabbits, the sperm cells where collected using an artificial vagina. The study was carried out in the university of benin health services department. Smear were prepared from the wister rats and rabbits under study; the smears were dried at room temperature and fixed in methyl alcohol.
In the first part of the study, smears were stained with (giemsa stain), giemsa and for (7) seven minutes and it was latter rinsed in buffered normal saline, then for the second stain it was stained in hibiscus flower extract popularly called zobo for (3) minutes and it was rinsed then blotted out and left to air dry.
Result
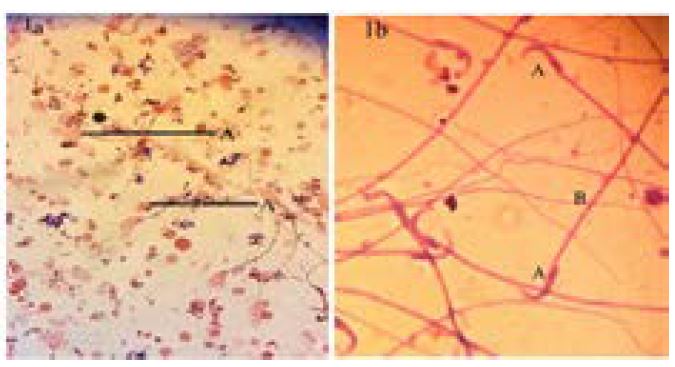
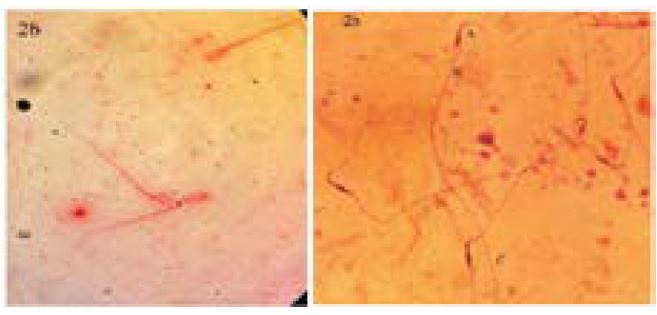
Head, middle piece and tails of spermatozoa were seen clearly withge. Acrosome core rate was extremely good in the form of light and dark pinkish (condensation). The stain was homogeneously distributed on the head and dark pinkish color was seen. The middle piece and tail were seen clearly and can be evaluated very well (figure 1a and b).The ideal image was obtained by staining spermatozoa with giemsa and eosin. The condensation of the head can be chosen by the whitish blue color. Condensation and morphology of the head can be selected clearly (figure 2 a and b).
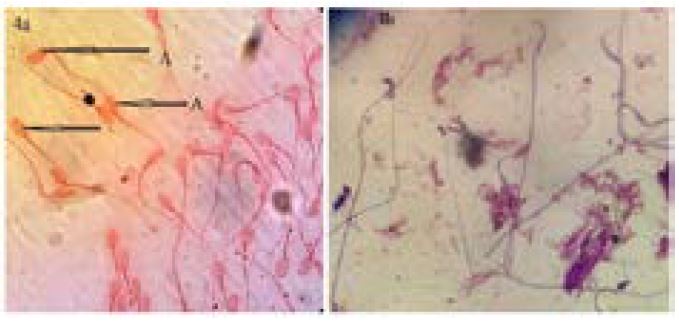
The spermatozoa were stained dark blue purple with giemsa and hibiscus flower extract (zobo) with increased concentration of giemsa stain without rinsing in buffered normal saline (figure 4b). Head morphology and condensation could be seen very well but the middle piece and the tail did not seem clear.
Discussion
Assessment of spermatozoa morphology is an important parameter in the diagnosis of infertilemen. Due to the use of increasing amounts of ivf (invitro fertilization) techniques, studies are also focused on the morphology of spermatozoa and its significant role infertilization. Evaluation can be done, staining spermatozoa with a variety of methods from the fresh semen by electron, fast-contrast and light microscope. There are many staining methods to evaluate the morphology of spermatozoa [5]. Garcia-herreros et al.[6] used hemaklor, harris haematoxylin and panoptic fast staining techniques to make standardization of the sampling methods, sample preparation and staining for the head of the spermatozoa with computer- assisted morphometric analysis (casma). They concluded that haematoxylin and hemaklorun are the best staining methods for evaluation of sperm heads.
Soler et al. [7] compared 3 different dyemethods for assessment of spermatozoa morphometry with computerized integrated semen analysis system[7]. They evaluated 200 spermatozoa cells stained with hemacolor, diff-quik and harris haematoxylin. While they found all techniques were also useful in that used in staining of spermatozoa but diff-quick stain showed a significant difference.
Tb is used in the evaluation of the nuclear chromatin of spermatozoa by identifying the absence or rupture of disulfide bonds. Beletti& mello [8] examined relationship between spermatozoa morphology and spermatozoa chromatin condensation with Tb and fuel genre action. Tb is an effective stain for the evaluation of changes in spermatozoa chromatin. Our study is compatible with the knowledge of the literature. Tb dyestaining method is a simple method and allow simultaneous evaluation for morphology and condensation of spermatozoa. Tb is an ideal dye for routine use in this area.
Morel et al. [9] randomly selected 47 patients applied for semen analysis, they examined the diversity of common morphological disorders in the human spermatozoa among individuals. In order to show their connection with the nuclear maturity, sperms stained with an ilineblue, spermatozoa morphology defects differences seen between individuals and they found a significant relationship between frequent of defects and degree of nuclear maturate. Our study is compatible with the knowledge of the literature. An ilineblue dye provides the ideal image to measure the rate of acrosome head. Condensation and morphology can be assessed together.
Tartaglione& ritta [10] examined the prognostic spermatological values of frozend is solved spermatozoa to estimate in vitro fertility. Estimating plasma membrane and acrosome integrity are important values for normal functions of spermatozoa, for this aim, they examined smears stained with trypan blue and giemsa. They concluded that these stains can be used for the prognosis of the fertility in the semen used for ivf
Our evaluation was consistent with the literature of giemsa and wright stains. Condensation and head morphology of spermatozoa were well-selectable. The middle piece and tail could be seen. An unexpected finding that tail and head completely stained pink in the some of the spermatozoa which had damaged head morphology. Further study was suggested to explain the reason. Spermatozoa morphology and condensation were clear and smooth with orangeg stain. But staining process was too long that’s why orange stain had no superiority.
Shorr technique is preferred in many laboratories due to the in sufficient relationship in the results of ivf. [11] evaluated decreased motility of spermatozoa in elderly men with shorr technique. The percentage of stained spermatozoa with normal and abnormal flagella’s was evaluated and found a negative correlation between the stained abnormal flagella’s and speed ratio and motility of the spermatozoa in elderly man..
Conclusively in our study, spermatozoa heads appeared as pinkish bulbs with the balance of concentration with time light pale pinkish coloured, middle sections and tails were stained dark pinkish .Our study was appeared to be compatible with the literature and as the result of our study. Spermatozoa middle section which is a rich section for mitochondria’s were revealed clearly and was displayed once again by the giemsa hibiscus extract stain which is proposed by this research as a simple and accessible stain for spermatozoal with wister rats and rabbits as a model.
Acknowledgement
I wish to register my profound gratitude to the national academy for the advancement of science, for the immense support in terms of grants, i also wish to thank all member and staff of the health services unit of the university of benin, benin city.
Conflict of interest
The were no conflicting interest during this study
References
- Kuster CE, Singer RS, Althouse GC (2004) Determining sample size for the morphological assessment of sperm. Theriogenology 61: 691-703.
- Menkveld R, Lacquet FA, Kruger TF, Lombard CJ, Sanchez Sarmiento CA, et al. (1997) Effects of different staining and washing procedures on the results of human sperm morphology evaluation by manual and computerised methods. Andrologia 29: 1-7.
- Mehta R H, Makwana S, Ranga G M, Srinivasan R J, Virk SS (2006) Prevalences of oligozoospermia and azoospermia in male partners of infertile couples from different parts of India. Asian J Androl 8: 89-93.
- Irez T, Kucur M, Is man K (2007) Semenanalysis. Andrology Laboratory Handbook. I?stanbul, Nobel Medical Publishing.
- Uven M, Can B, Saran Y (1998) Ultra structural Changes in the Spermatozoa of Infertile Men. Turkiye Klinikleri J Med Res 16: 103-105.
- Garcia-Herreros M, Aparicio IM, Baron FJ, Garcia-Marin LJ, Gil MC (1998) Standardization of sample preparation, staining and sampling methods for automated sperm head morphometry analysis of boar spermatozoa. Int J Androl 29: 553-563.
- Soler C, Gadea B, Soler AJ, Fernandez-Santos MR, Esteso MC, et al. (2005) Comparison of three different staining methods for the assessment of epididymal red deer spermatozoa morphometry by computerized analysis with ISAS. Theriogenology 64: 1236-1243.
- Beletti ME, Mello ML (2004) Comparison between the toluidine blue stain and the Feulgen reaction for evaluation of rabbit sperm chromatin condensation and their relationship with sperm morphology. Theriogenology 62: 398-402.
- Morel F, Mercier S, Roux C, Elmrini T, Clavequin MC, et al. (1998) Inter individual variations in the disomy frequencies of human spermatozoa and their correlation with nuclear maturity as evaluated by aniline blue staining. Fertil Steril 69: 1122-1127.
- Tartaglione CM, Ritta MN (2004) Prognostic value of spermatological parameters as predictors of in vitro fertility of frozen-thawed bull semen.Theriogenology 62: 1245-1252.
- Henkel R, Maass G, Schuppe HC, Jung A, Schubert J, et al. (2005) Molecular aspects of declining sperm motility in older men. Fertil Steril 84: 1430-1437.
- Anafarta K, Bedük Y, Ar?kan N ( 2007) Principles of Urology.Ankara, Sun Publishing Pp222.
- Avc? B (2006) Different fix at ionprotocols with chromatin condensation of spermatozoa with sperm chromatin anomaly evaluation protocols. Uludag Univ. Med. Fac. J 32: 55-9.
- Gago C, Perez-Sanchez F, Yeung CH, Tablado L, Cooper TG, et al (1998) Standardization of sampling and staining methods for the morphometric evaluation of sperm heads in the Cynomolgus monkey (Macaca fascicularis) using computer-assisted image analysis. Int J Androl21: 169-176.
- Hammadeh ME, Zeginiadov T, Rosenbaum P, Georg T, Schmidt W, et al. (2001) Predictive value of sperm chromatin condensation (aniline blue staining) in the assessment of male fertility.Arch Androl 46: 99-104.
- Michael AY, Drejer JO, Bagger PV, Detlefsen GU, Stakemann G (1987) Complete staining of human spermatozoa and immature germ cells combined with phase contrast microscopy. Arch Androl 19: 217-221.
- Milingos S, Comhaire FH, Liapi A, Aravantinos D (1996) The value of semen characteristics and tests of sperm function in selecting couples for intra-uterine in semi nation. Eur J Obstet Gynecol Reprod Biol 64: 115-118.
- Palermo G, Joris H, Devroey P, Van Steirteghem AC (1992) Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340: 17-18.
- Root Kustritz MV, Olson PN, Johnston SD, Root TK (1998) The effects of stains and Investigators on assessment of morphology of canine spermatozoa. J Am Anim Hosp Assoc 34: 348-352.
- Sanchez R, Villagran E, Risopatron J, Celis R (1994) Evaluation of nuclear maturity in human spermatozoa obtained by sperm- preparation methods. Andrologia 26: 173-176.
- Spira A (1986) Epidemiology of human reproduction. Hum Reprod 1: 111-115.
- WHO 1999.Laboratory Manual for the Examination on Human Semen and Spermatozoa-Cervical MucuInteraction.Cambridge, Cambridge University Press.