Short Communication
Lisa Kitasato1*, Minako Yamaoka-Tojo2,3, Ryota Kakizaki3, Teruyoshi Nemoto3, Sayaka Namba3, Takehiro Hashikata3, Takuya Hashimoto3, Ryo Kameda1, Takao Shimohama1, Taiki Tojo1, and Junya Ako1,3
1Department of Cardiovascular Medicine, Kitasato University School of Medicine, Sagamihara, Japan
2Department of Rehabilitation, Kitasato University School of Allied Health Sciences, Sagamihara, Japan
3Kitasato University Graduate School of Medical Sciences, Sagamihara, Japan
Corresponding author
Dr. Lisa Kitasato, M.D., Ph.D, Kitasato University School of Medicine, Department of Cardiovascular Medicine, 1-15-1 Kitasato, Minami-ku, Sagamihara, Kanagawa 252-0373, Japan; Tel: +81-42-778-8111; Fax: +81-42-778-9696; E-mail: lisa@med.kitasato-u.ac.jp
Received Date: 28th April 2015
Accepted Date: 15th May 2015
Published Date: 22nd May 2015
Citation
Kitasato L, Yamaoka-Tojo M, Kakizaki R, Nemoto T, Namba S, Hashikata T, HashimotoT, Kameda R, Shimohama T, Tojo T, and Ako J (2015) Endothelial Cell Survival under High Glucose Condition: Effect of Rivaroxaban. Enliven: Clin Cardiol Res 2(1): 003.
Copyright
@ 2015 Dr. Lisa Kitasato. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Endothelial injury is regarded as a key early event in the development of atherosclerosis. High level of glycemic excursion has been reported to play an important role in the pathogenesis of atherosclerosis. Recently, proteinase-activated receptor-2 (PAR2), activated by factor (F) Xa, has been shown to play a pivotal part in diabetes-induced endothelial dysfunction. In the present study, we have investigated the effect of rivaroxaban, a direct FXa-inhibitor by analyzing cellular biological function of human umbilical vein endothelial cells (HUVECs) in a glycemic excursion-model.
Methods: HUVECs were starved in 1% fetal calf serum media with 0.5% glucose for 4 hours and treated with or without rivaroxaban 0.1μg/mL, followed by stimulation of glucose in concentration of 0.5 mM, 5mM, 15mM and 30 mM. Cell viability was measured after 3 hours of stimulation by glucose using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Cell without rivaroxaban treatment and then stimulated with 0.5mM glucose was used as control. The high glucose-induced cell apoptosis was also evaluated with or without pre-treatment of rivaroxaban based on TUNEL method, using fluorescein-dUTP to label DNA strand breaks. All TUNEL-positive HUVECs in whole mount slide were counted. The derivatives of reactive oxygen metabolites (dROMs) were measured 45 minutes after the glucose stimulation to evaluate the production of reactive oxygen species (ROS).
Results: In rivaroxaban treated group, there was a significant increase in cell viability in high-glucose condition (113% in 0.5mM, 103% in 5 mM, 125% in 15 mM compared with cells without rivaroxaban pre-treatment, respectively). There was a significant increase in TUNEL positive cells in a glucose concentration dependent manner without rivaroxaban treatment; however, rivaroxaban pre-treatment significantly inhibited cell apoptosis (75% decrease in 15 mM glucose-stimulated-cells). There was a significant increase in ROS production in glucose-stimulated HUVECs without rivaroxabantreatment, which was also inhibited when pre-treated with rivaroxaban (30% decrease compared without rivaroxaban-pretreatment) (p< 0.05).
Conclusions: Rivaroxaban increases endothelial cell viability and inhibits apoptosis induced by high level of glycemic excursion, possibility through ROS signaling. These results suggest that rivaroxaban may be effective in the prevention of atherosclerosis.
Keywords
Rivaroxaban; Human Umbilical Vein Endothelial Cells; Reactive Oxygen Species; Apoptosis; High-Glucose
Introduction
Endothelial injury or the loss of proper endothelial function is often regarded as a key early event in the development of atherosclerosis [1]. Diabetes represents an example of endothelial dysfunction coupled to cardiovascular inflammation. Several studies reported that duration of diabetes and glucose fluctuations exhibited a specific triggering effect on oxidative stress, indicating a close link between metabolic impairment and endothelial injury [2-6]. Although peripheral arterial disease results from atherosclerotic narrowing of the blood vessel lumen, endothelial dysfunction has been shown to play a role in the progression of this disease. Further, these processes are enhanced by the metabolic disturbances associated with diabetes and denervation of the smooth muscle of the tunica media of arteries caused by the diabetic neuropathy.
Proteinase-activated receptor-2 (PAR2), a seven transmembrane domain G-protein coupled receptor profoundly localized in the vasculature, especially in endothelial cells [6-10] are activated by factor (F) Xa. PAR2 has been shown to be involved in cardiovascular function [11, 12]. We also have previously demonstrated that FXa was associated with cardiac fibrosis [13]. Furthermore, it has been recently reported that PAR2 play a pivotal role in diabetes-induced endothelial dysfunction by up-regulating the expression or production of TNF-? and activating NAD(P)H oxidase [14].
Rivaroxaban is a direct FXa inhibitor which binds directly to the Xa active site, blocking its activity [15]. Rivaroxaban is 100,000-fold more selective for FXa than for other biological proteases such as thrombin, plasmin, factor VIIa or factor IXa, and is widely used as treatment of venous thromboembolism and stroke prophylaxis in atrial fibrillation [16-18]. We also have reported that rivaroxaban did not only effect to interrupt the blood coagulation cascade but was effective to inhibit activation of cardiac fibroblast via reductions of major inflammatory signal cascades involved in cardiovascular disease [19]. In the present study, we have investigated the effect of rivaroxaban, by analyzing cellular biological function of human umbilical vein endothelial cells (HUVECs) in a glycemic excursion-model.
Methods
Reagents
Rivaroxaban was obtained from Toronto Research Chemicals (Ontario, Canada). MTT assay kits were purchased from Roche (Basel, Switzerland), MEBSTAIN apoptosis TUNEL Kit Direct from MBL (Nagoya, Japan). All other reagents used were of analytical grade.
Cell Culture
HUVECs obtained from Lonza Group Ltd. (Basel, Switzerland) were cultured in endothelial basal medium supplemented with 2% fetal bovine serum, 0.4% bovine brain extracts, 10 ng/ml human epidermal growth factor and 1 ?g/ml hydrocortisone according to the supplier?s instructions. Rivaroxaban 0.1?g/mL treatment was carried out in a medium with 1% fetal bovine serum, lacking bovine brain extracts, epidermal growth factor and hydrocortisone.
Proliferation Assay (MTT Assay)
Cells seeded at a density of 104cells/well in 96-well plates were treated for 4 hours with rivaroxaban 0.1?g/mLfollowed by stimulation of glucose in concentration of 0.5mM, 5mM 15mM and 30mM. Cell survival was determined after 3 hours of glucose stimulation using MTT assay as described previously [20]. Cell without rivaroxaban treatment was used as control.
ROS Production
The derivatives of reactive oxygen metabolites (dROMs) were measured 45 minutes after the glucose stimulation to evaluate the production of reactive oxygen species (ROS) by a photometric assay that measures the total oxidant capacity (mainly dependent on the total amount of hydroperoxides, a class of reactive oxygen metabolites) of a sample against N,N-diethyl paraphenylenediamine.
HUVEC apoptosis Assay
Apoptotic cells were detected with a MEBSTAIN Apoptosis Kit Direct. Briefly, HUVECs cells were fixed with 4% paraformaldehyde. The 3?-OH DNA ends generated by DNA fragmentation were then nick end-labeled with fluorescein isothiocyanate-dUTP, and the nuclei were stained with Propidium Iodide (PI).
Apoptotic cells were observed under a laser microscope (Olympus), and the measurement of apoptosis was calculated as a percentage of apoptotic nuclei versus total nuclei of HUVECs.
Statistical Analysis
Data were expressed as the mean ± SE. The significance for the difference among groups was analyzed with JMP10.0 (SAS institute company, NC, USA) by one-way ANOVAs. Differences were considered to be statistically significance at value of P < 0.05.
Results
Rivaroxaban Treatment Increased Cell Viability in High-Glucose Condition
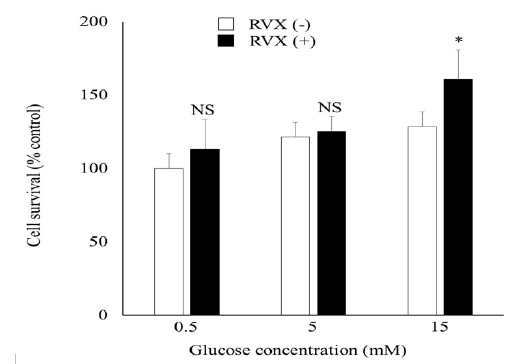
To analyze the effect of rivaroxaban on HUVEC viability, we evaluated a MTT assay. As shown in (Figure 1), rivaroxaban treatment increased cell viability in high-glucose condition (113% in 0.5mM, 103% in 5 mM, 125% in 15 mM compared with cells without rivaroxaban pre-treatment, respectively).
Figure 1: Cell Survival in HUVECs
Effect of RVX on glucose-stimulated cell survival HUVECs. HUVECs was treated with rivaroxaban (RVX) 0.1?g/mL followed by stimulation of glucose in concentration of 0.5mM, 5mM 15mM and 30mM. Cell without RVX pre-treatment was used as control. Results are shown as mean +/- SE for 3 independent experiments. *P < 0.05 compared with control.
HUVEC, human umbilical vein endothelial cell; RVX, rivaroxaban.
Rivaroxaban Treatment Inhibited Cell Apoptosis in High-Glucose Condition
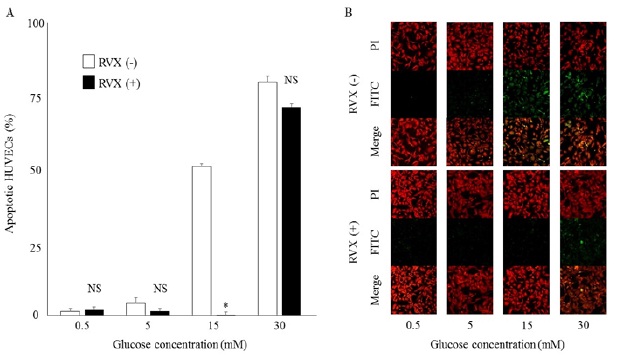
HUVEC apoptosis may lead to endothelial dysfunction and contribute to the progression of atherosclerosis; we therefore investigated the effect of rivaroxaban on HUVEC apoptosis under high glucose condition. This was examined by TUNEL and PI counter staining. There was a significant increase in TUNEL positive cells in a glucose concentration dependent manner without rivaroxaban treatment; however, rivaroxaban pre-treatment significantly inhibited cell apoptosis (75% decrease in 15 mM glucose-stimulated-cells, (Figure 2 A, B)).
Figure 2: Detection of Apoptosis by TUNEL Assay in HUVECs.
A: The presence of apoptotic HUVECs after treatment of RVX followed by glucose stimulation. The data represent the average +/- SE.
B: TUNEL assay of HUVECs pre-treated with or without RVX in high-glucose condition. Cells were treated with or without RVX as noted for hours, followed by glucose stimulation for 3 hours and labeled with fluorescein-12-dUTP and PI counterstaining.
HUVEC, human umbilical vein endothelial cell; RVX rivaroxaban; FITC, fluorescein isothiocyanate-dUTP; PI, Propidium Iodide.
Rivaroxaban Treatment Inhibited ROS Production in High-Glucose Condition
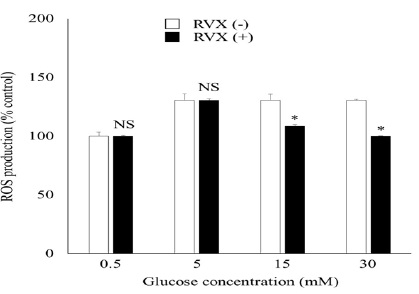
We next determine if rivaroxaban can inhibit high-glucose induced ROS production. As shown in (Figure 3), there was a significant increase in ROS production in glucose-stimulated HUVECs without rivaroxaban-treatment, which was also inhibited when pre-treated with rivaroxaban (22% and 30% decrease in 15mM and 30mM glucose stimulated-cells compared to cells without rivaroxaban-pretreatment).
Figure 3: ROS Production in HUVECs
Effect of RVX on HUVEC ROS production after 45 min of glucose stimulation. Graphs represent averaged data. Data are expressed as mean +/- SE for 3 independent experiments. *P < 0.05 compared with control.
HUVEC, human umbilical vein endothelial cell; RVX, rivaroxaban; ROS, reactive oxygen species.
Discussion
In the present study, we provide evidence that rivaroxaban could increase HUVEC viability in high level of glycemic excursion. We also found that rivaroxaban inhibited cell apoptosis and ROS production of HUVEC under high-glucose condition.
It is well known that duration of diabetes and glucose fluctuations are the most important risk factors for the development of atherosclerosis, by exhibiting a specific triggering effect on oxidative stress which leads to endothelial injury [2-7]. Cell survival and apoptosis in response to endothelial dysfunction are important features in atherosclerosis [21]. Furthermore, it has been recently reported that the PAR2 play a key role in pathologies leading to endothelial dysfunction in type 2 diabetes by up-regulating the expression or production of TNF-? and activating NAD(P)H oxidase [14]. Consistent with these previous results, the present study demonstrated that rivaroxaban increase cell viability, inhibit cell apoptosis under high level of glycemic excursion condition in cultured HUVEC. This study also revealed that the level of intracellular ROS was elevated in high glucose concentration-treated HUVECs and suggested that this increase of ROS is inhibited by rivaroxabanpre-treatment. Enhanced oxidative stress interacts with TNF-?, NF-?B, AGE/RAGE and ox-LDL/LOX-1inducing endothelial dysfunction in type 2 diabetes [22-24].
More studies are needed to identify other associated apoptotic signaling pathways after high glucose treatment, such as the NF-kappa B signal pathway [25].
Conclusion
Rivaroxaban increases HUVEC viability and inhibits apoptosis induced by high-glucose, possibility through ROS signaling. These results suggest that rivaroxaban may be effective in the prevention of atherosclerosis.
Competing Interests
Dr. Junya Ako was partly supported by speaking honorarium from Bayer (J.A), Dr. Minako Yamaoka-Tojo by International Grant from MSD K.K., Bayer Pharma, Daiichi-Sankyo, and Boehringer Ingelheim (M.Y.-T.). All other authors have nothing to disclose regarding this manuscript.
Acknowledgements
This work was partly supported by Grant-in Aid for Research Project, from Kitasato University School of Allied Health Sciences (M.Y.-T.).
We would like to thank Miss Kazumi Nakazato, Dr. Miki Sakamoto, Dr. Yuki Ikeda for their technical assistance.
References
- Gonzalez-Gay MA, Gonzalez-Juanatey C (2012) Inflammation, endothelial function and atherosclerosis. Arthritis Res Ther 19 : 2504-2520.
- Monnier L, Mas E, Ginet C, Michel F, Villon L, et al. (2006) Activation of oxidative stress by acute fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 295: 1681-1687.
- Suzuki K, Olah G, Modis K, Coletta C, Kulp G, et al. (2011) Hydrogen sulfide replacementtherapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc Natl Acad Sci USA 108: 13829-13834.
- Morrish NJ, Stevens LK, Fuller JH, Jarett RJ, Keen H (1991) Risk factors for macrovascular disease in diabetes mellitus: the London follow-up to the WHO Multinational Study of Vascular Disease in Diabetics. Diabetologia 34: 590-594.
- Hu FB, Stampfer MJ, Solomon CG, Liu S, Willett WC, et al. (2001) The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med 161: 1717-1723.
- Kitasato L, Tojo T, Hatakeyama Y, Kameda R, Hashikata T, et al. (2012) Postprandial hyperglycemia and endothelial function in type 2 diabetes: focus on mitiglinide. Cardiovasc Diabetol 11:79
- Cicala C, Pinto A, Bucci M, Sorrentino R, Walker B, et al. (1999) Protease-activated receptor-2 involvement in hypotension in normal and endotoxemic rats in vivo. Circulation 99: 2590-25907
- D'Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, et al. (1998) Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem 46: 157-164
- Hwa JJ, Ghibaudi L, Williams P, Chintala M, Zhang R, et al. (1996) Evidence for the presence of a proteinase-activated receptor distinct from the thrombin receptor in vascular endothelial cells. Circ Res 78: 581-588.
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R (2001) Proteinase-activated receptors. Pharmacol Rev 53: 245-282.
- El-Daly M, Saifeddine M, Mihara K, Ramachandran R, Triggle CR, et al. (2014) Proteinase-activated receptors 1 and 2 and the regulation of porcine coronary artery contractility: a role for distinct tyrosine kinase pathways. Br J Pharmacol 171: 2413-2425.
- Gieseler F, Ungefroren H, Settmacher U, Hollenberg M, Kaufmann R (2013) Proteinase-activated receptors (PARs) - focuson receptor-receptor-interactions and theirphysiological and pathophysiological impact. Cell Commun and Signal 11: 86
- Kitasato L, Yamaoka-Tojo M, Hashikata T, Ishii S, Kameda R, et al. (2014) Factor Xa in mouse fibroblasts may induce fibrosis more than thrombin. Int Heart J 55: 357-361.
- Park Y, Yang J, Zhang H, Chen X, Zhang C (2011) Effect of PAR2 in regulating TNF-? and NAD(P)H oxidase in coronary arterioles in type 2 diabetic mice. Basic Res Cardiol 106: 111-123.
- Laurent M, Joimel U, Varin R, Cazin L, Gest C, et al. (2014) Comparative study of the effect of rivaroxaban and fondaparinux on monocyte's coagulant activity and cytokine release. Exp Hematol Oncol 3: 30.
- Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, et al. (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 358: 2765-2775.
- Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, et al. (2008) Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 372: 31-39.
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, et al. (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365: 883-891.
- Hashikata T, Yamaoka-Tojo M, Namba S, Kitasato L, et al. (2015) Rivaroxaban inhibits angiotensin II-induced activation in cultured mouse cardiac fibroblasts through the modulation of NF-?B pathway. Int Heart J 56.
- Versteeg HH, Spek CA, Richel DJ, Pepelenbosch MP (2004) Coagulation factors VIIa and Xa inhibit appotosis and anoikis. Oncogene 23: 410-417.
- Gimbrone MA Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G (2000) Endothelial dysfunction, hemodynamic forces, and atherogenesisa. Ann N Y Acad Sci 902: 230-239.
- Gao X, Zhang H, Schmidt AM, Zhang C (2008) AGE/RAGE produces endothelial dysfunction in coronary arterioles in Type 2 diabetic mice. Am J Physiol Heart CircPhysio l 295: 491-498
- Yang J, Park Y, Zhang H, Xu X, Laine GA, et al. (2009) Feed-forward signaling of TNF-alpha and NF-kappaB via IKK-beta pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 296: 1850-1858.
- Zhang C (2008) The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol 103: 398-406.
- Zheng X, Zhu S, Chang S, Cao Y, Dong J, et al. (2013) Protective effects of chronic resveratrol treatment onvascular inflammatory injury in steptozotocin-induced type 2diabetic rats: role of NF-kappa B signaling. Eur J Pharmacol 720: 147-157