Retrospective Study
Dina R. Bassiouny1, Mahmoud A. El-Baz1, Tawakol M. Gamil1, Nazem Shams2, Nadia Ismiil3,4, Valerie Dube3,4, Guangming Han3,4, Matthew Cesari3,4, Fang-I Lu3,4, Elzbieta Slodkowska3,4, Sherine Salama3, Hak Fai Chiu3, Magda Naeim3, Nim Li3, Hadas Moshonov3, Sharon Nofech-Mozes3,4, Mahmoud Khalifa3,4
1Department of Pathology, Mansoura University, Egypt
2Surgical Oncology Department, Oncology Centre, Mansoura University, Egypt
3Sunnybrook Health Sciences Centre, Toronto, On, Canada
4Department of Laboratory Medicine and Pathobiology, University of Toronto, On, Canada
Corresponding author
Dina Reda Bassiouny, MD, Department of Pathology, Sunnybrook Health Sciences Centre, Canada; Tel: 416 480 6100 ext. 3130; E-mail: Dina.Bassiouny@Sunnybrook.ca
Received Date: 01st July 2015
Accepted Date: 30th July 2015
Published Date: 07th August 2015
Citation
Bassiouny DR, El-Baz MA, Gamil TM, Shams N, Ismiil N, et al. (2015) Endometriosis associated ovarian cancer, does direct transition matter? Enliven: Gynecol Obstet 2(3): 006
Copyright
@ 2015 Dr. Dina R. Bassiouny. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Abstract:
Endometriosis associated ovarian carcinoma (EAOC) is currently receiving attention due to the controversy about its biological behavior. It is not clear
whether tumors with demonstrable transition of benign to malignant lining of ovarian endometriosis differ from ovarian carcinomas with associated
endometriosis in cases where direct transition is not evident. This study examined the association between the relationship of endometriosis and ovarian cancer and histopathologic characteristics, p53 status and outcome.
Methods:
140 patients with EAOC with available tissue met the inclusion criteria. Patients were categorized as EAOC type I when direct transition of benign to
malignant lining was identified in ovarian endometriosis and EAOC type II when ovarian endometriosis was present but no direct transition to carcinoma
was demonstrated. Age, histologic type, FIGO stage and disease free survival were compared between the groups. P53 status was determined by
immunohistochemistry using tissue microarray.
Results:
Seventy one (51.07%) patients had EAOC type I while 69 (49.6%) patients had EAOC type II. There was a significant difference in cell type composition
between the groups. The most common histologic type in EAOC type I was endometrioid carcinoma (35, 49.3%) followed by clear cell carcinoma (29,
40.8%) and serous carcinoma (7, 9.9%). The most common histologic type in EAOC type II was serous carcinoma (35, 50.7%) followed by endometrioid
carcinoma (20, 29%), clear cell carcinoma (9, 13%) and mucinous carcinoma (5, 7.2%), P < 0.001. Of 140 patients with EAOC, 139 tumor tissue
specimens were identified on the TMAs. Mutant p53, either overexpressed or null phenotype, was detected in 16/71 (22.5%) patients with EAOC type I
vs. 33/68 (48.5%) patients with type II, P=0.003. However when p53 status was compared in the two groups by tumor histology and grade, there was no
significant difference between EAOC type and P53 status. EAOC type I cases were more likely to present at a lower stage. DFS was not associated with
EAOC type but was significantly decreased in cases with mutant p53.
Conclusion:
In this single institution cohort, the outcome of patients with ovarian carcinoma was related to the histological type and stage. However, the type of
EAOC was not independently predictive of outcome. Biological differences such as in p53 status, stage at diagnosis and outcome reflect the higher
proportion of serous and high grade endometrioid carcinoma in EAOC type II.
Keywords
Ovarian Carcinoma; Endometriosis; p53; Immunohistochemistry
Introduction
Endometriosis, the presence of endometrial tissue outside the uterine fundus, is a common condition affecting about 15% of women. Malignant transformation in endometriosis has been noted as early as 1925 by Sampson [1]. Endometriosis associated ovarian carcinoma (EAOC) is currently receiving attention due to the controversy about its biological behavior. Previous studies noted that the most common histological types associated with endometriosis are endometrioid and clear cell carcinoma whereas the most common histologic type of ovarian carcinoma not associated with endometriosis is high grade serous carcinoma. It is unknown whether the tumor biology and outcome in EAOC reflect the difference in histological cell type or whether the outcome is different even within the same histologic type. Some studies have shown better survival in EAOC patients [2,3] while others showed no difference between EAOC vs. non-EAOC group. The criteria for EAOC that were suggested by Sampson and Scott continue to be used by more recent investigators. These include: 1) the coexistence of endometriosis and carcinoma in the same ovary 2) presence of tissue resembling endometrial stroma surrounding characteristic epithelial glands 3) exclusion of a metastatic tumor to the ovary and 4) presence of benign endometriosis histologically close to the tumor [9,10]. Subsequently, epithelial ovarian carcinomas associated with endometriosis were further defined as carcinomas that were found in surgical specimens when not in close continuity with the endometriosis [1,2,5,12-15].
P53 is a tumor suppressor gene located on chromosome 17p13 that was found to be mutated in 50% of advanced cases of ovarian cancer [16]. P53 expression is induced as a result of oncogene activation, DNA damage and hypoxia and its activation results in apoptosis or cell cycle arrest. Normally, p53 is regulated through its interaction with MDM2 via a negative feedback mechanism. P53 activity can be inhibited by viral protein such as human papilloma virus E6, adenovirus E1A, simian virus 40 large T antigen and hepatitis B x antigen [17]. P53 protein also plays an essential role in apoptosis through decreasing the expression of antiapoptotic genes and increasing the expression of proapoptotic genes [18].
Two main types of p53 mutations are well characterized, one results from loss of function of both alleles leading to overexpression in order to compensate for the loss of its function [19]. Such overexpression is due to missense mutation in exons 5-8, leading to accumulation of the mutated protein that can be detected by immunohistochemistry (IHC). Another mutation occurs outside these exons and leads to the formation of truncated dysfunctional protein that cannot be detected by IHC (no staining) which is referred to as p53 null phenotype [20,21]. Wild-type p53 protein is relatively unstable and has a very short half-life. The typical wild-type p53 IHC pattern is patchy with intermingled negative and positive cells with variable intensity [22].
Immunohistochemical analysis of p53 expression is used as a surrogate for mutational status [23,24]. The clinical significance of p53 overexpression in patients with ovarian cancer was investigated but the results are inconsistent [25,26]. Many studies demonstrated the relevance of p53 to the tumorigenesis of ovarian epithelial tumors and its importance as a biologic marker [27-30]. In this study, we identified patients with EAOC and categorized them according to the relationship between the endometriosis and the cancer. We investigated IHC expression of p53 in ovarian carcinoma arising directly in or associated with endometriosis and correlated its expression with patient’s characteristics and biological behavior.
Methods and Materials
Patients
After obtaining the institutional ethics review board approval, the database of the Department of Anatomic Pathology, Sunnybrook Health Sciences Centre (Copath™) was searched for in-house specimens to identify ovarian cancer cases between 2000-2013. Pathology reports were screened to identify patients with any carcinoma involving the ovary. Results were filtered to patients who underwent at least a cystectomy procedure. After exclusion of metastatic tumors, cases with indeterminate histological tumor type or controversial site of origin and cases with minimal microscopic disease post neoadjuvant chemotherapy (NAT), 702 (58.5%) patients with primary carcinoma were available for the study. Follow-up data were collected from the electronic patient record including time to recurrence and last date of follow-up. Data elements were recorded in an institutional cancer database (Biomatrix).
Histopathology
All available Hematoxylin and Eosin (H & E) stained slides were reviewed by a group of gynecologic pathologists and one of the research fellows (DB & HFC) to verify the diagnosis and complete all the pathology data elements according to the (2010) CAP Cancer Checklist [31]. The histological type was determined based on the WHO criteria and any discrepancy was resolved by consensus using multiheaded microscope and in few difficult cases IHC was used at the discretion of the review team to verify diagnoses. Staging was recorded for each case according to the international Federation of Gynecology and Obstetrics (FIGO) staging system [32].
The presence of endometriosis whether ovarian or extra-ovarian was identified on H&E sections by the presence of glandular epithelium accompanied by endometrial stroma, and/or hemorrhagic stroma or histiocytes containing hemosiderin within a thick fibrous wall [33]. Endometriosis associated ovarian carcinoma (EAOC) was defined by the presence of endometriosis and carcinoma in the same ovary. As part of the pathology review process, cases were categorized based on their type of association with endometriosis.
Cases with tumors arising directly in endometriosis where transition of benign to malignant lining could be demonstrated were recorded as EAOC type I (Figure 1) and cases where direct transition was not seen and ovarian endometriosis was noted away from the tumor in another focus but in the same ovary were recorded as EAOC type II. The term transition refers to morphologic continuity of the ovarian carcinoma with the benign endometriotic epithelium and not molecular transformation. Given the absence of a robust adjunct tool to distinguish between the two scenarios, classification was based on morphologic assessment.
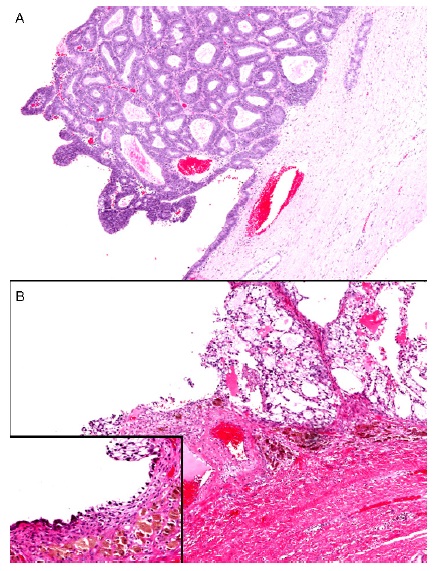
Figure 1: EAOC type I showing the direct transition between the endometriosis and the ovarian carcinoma
A. Low grade endometrioid carcinoma arising in endometriotic cyst
B. Clear cell carcinoma arising in endometriotic cyst with the inset showing the endometrial lining of the ovarian cyst with underlying hemosiderin
Among 702 patients with primary ovarian carcinoma, 168 patients (23.9%) were diagnosed as primary ovarian carcinoma either arising in or associated with endometriosis. Of them, 140 patients were included in the study after exclusion of mixed histological cell types (17 cases) and cases with undetermined histological types post NAT (3 cases) and cases with tiny tumor tissue unsuitable for TMA coring (8 cases). Representative blocks of the ovarian carcinoma were selected for TMA construction.
Tissue Microarray (TMA) Construction
Representative tumor tissue was arrayed in duplicates of 1mm cores using the tissue arrayer (Beecher Instruments, Silver Spring, MD).
IHC
Formalin-fixed-paraffin embedded TMA blocks were sectioned at 4µm thickness and the slides were baked for 30 minutes at 60± 2ºC before staining. All TMA slides were stained with a prediluted p53 mouse monoclonal antibody (DO-7) obtained from Ventana Medical, US system using Bench Mark ULTRA Autostainer. Antigen retrieval was carried out with CC1 for 76 min. Slides were incubated for 32 min at room temperature with the primary antibody. Staining was detected with ultra view universal DAB detection system. A colon adenocarcinoma that overexpressed p53 was used as positive control.
TMA slides were scanned using Aperio ScanScope XT slide scanner at 20 x resolutions. A set of 50 cases of consecutive serous carcinomas was used to train the primary readers (DB and HFC) who scored independently the study cases. A senior pathologist reviewed any discordance and 10% of all the cases.
IHC for p53 was interpreted according to a 3 tiered system: Null phenotype: no staining or very weak nuclear staining in less than 5%, overexpressed: strong nuclear staining in more than 75% of contiguous tumor cells and wild type pattern: nuclear staining with variable intensity and patchy distribution (intermingled brown and blue nuclei) [34]. Both cores of each case were scored separately. For the analysis null phenotype and overexpression patterns were referred to as mutated p53. For sections with no tumor, a score of "not available" was given. In case of discordance between the two cores, a full section slide from the tumor was re-stained.
Outcomes
Disease free survival (DFS) was determined as the time from the date of surgery to the date of first evidence of recurrence based on imaging or clinical assessment or to the last date of follow up in case of no recurrence.
Statistics
A descriptive analysis was performed using IBM SPSS software program (version 22, IBM Corp. Armonk, NY, United States) to compare between the groups. T test was used to compare the age between the groups. Chi square-test was used to compare the categorical variables between the groups. Survival analysis was performed using SAS software. Kaplan-Meier (KM) survival analysis was performed to compare the estimated mean and median disease free survival time (in months) along with its standard error (SE) and 95% confidence interval (CI). Log-Rank test was used to compare DFS between the groups. Cox-regression was used to assess the effect of the groups and the subgroups on the hazard of recurrence. Statistical significance was set at P-value < 0.05.
Results
Ovarian endometriosis was identified in 168/702 (23.9%) while extra-ovarian endometriosis was identified in 132/702 (18.8%). Patients with EAOC had more extra-ovarian endometriosis than patients with non-endometriosis associated ovarian carcinoma (69 patients, 41.1% vs 63 patients, 11.8%, respectively); P < 0.001.
After exclusion of mixed cell types and undermined histologic types post NAT, 140 patients with EAOC were included in the study. EAOC type I comprised 10.1% while EAOC type II comprised 9.8% of the whole population study.
Among 140 patients with EAOC, 139 tumor tissue specimens were identified on the TMAs. Endometrioid carcinoma was the most common histologic type (55, 39.6%), followed by serous carcinoma (42, 30.2%), clear cell carcinoma (38, 27.3%) and mucinous carcinoma (5, 3.5%). 71/140 (51.07%) patients had carcinoma arising in endometriosis (EAOC type I) while 69/140 (49.6%) patients had endometriosis in association with ovarian carcinoma (EAOC type II). Extra-ovarian endometriosis was identified in 40/71, 56.3% of patients with EAOC type I vs 22/69, 31.9% of patients with EAOC type II. The mean age of patients at presentation was 55.5±11.8 years in EAOC type I compared with 58.5±11.8 years in EAOC type II, P= 0.1.
There was a significant difference in the proportion of histologic types among the groups. The most common histologic type in EAOC type I was endometrioid carcinoma (35, 49.3%) followed by clear cell carcinoma (29, 40.8%) and serous carcinoma (7, 9.9%). However, the most common histologic type in EAOC type II was serous carcinoma (35, 50.7%) followed by endometrioid carcinoma (20, 29%), clear cell carcinoma (9, 13%) and mucinous carcinoma (5, 7.2%), P < 0.001.
In this series, 36 (25.8%) carcinomas were classified as low grade according to Shimizu- Silverberg Grading system [35], including: 26/55(47.3%) of endometrioid carcinomas, 5/42 (11.9%) serous carcinomas and 5/5 (100%) mucinous carcinoma. 18/71(25.4%) patients had low grade carcinoma among EAOC type I versus 18/69 (26.1%) patients among EAOC type II, P= 0.9.
P53 was overexpressed in 26/139 cases: 18 serous carcinoma (69.2%), 7 endometrioid carcinoma (26.9%) and 1 clear cell carcinoma (3.8%) but none of mucinous carcinomas. Null phenotype complete loss of nuclear staining was detected in 23 cases; 13 serous carcinoma (56.5%), 7 endometrioid carcinoma (30.4%), 2 clear cell carcinoma (8.7%) and 1 mucinous carcinoma (4.3%). The case of low grade mucinous carcinoma that demonstrated null phenotype using TMA was retested on full section and the result was confirmed. Figure 2 illustrates examples of different p53 expression patterns.
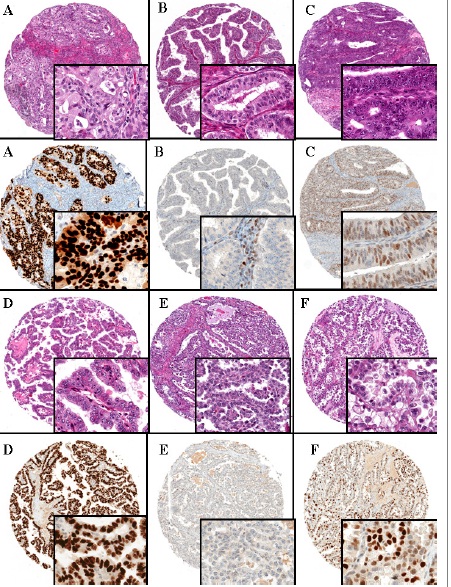
Figure 2: Paired H&E and p53 Immunohistochemical staining of ovarian carcinoma tissue microarrays
A. High grade endometrioid carcinoma with p53 overexpression
B. High grade endometrioid carcinoma with null phenotype. p53
staining is completely absent in the neoplastic cells but weakly positive in stromal cells (internal positive control)
C. High grade endometrioid carcinoma with wild-type pattern of p53 expression. There is partial labeling in the neoplastic cells with variable intensity
D. Clear cell carcinoma, p53 overexpression
E. Clear cell carcinoma, with null phenotype
F. Clear cell carcinoma, with wild-type pattern of p53 expression
There was a very high level of concordance between the two cores arrayed from each tumor. The case with discordant scoring was retested on full section and the overall pattern was consistent with wild type pattern. This finding indicates that intratumoral heterogeneity if any is exceedingly rare. Mutant p53, either overexpressed or null phenotype, was detected in 16/71 (22.5%) patients with EAOC type I versus 33/68 (48.5%) patients with type II, P= 0.003.
Surgical staging was based on the FIGO staging system: 78 patients (55.7%) were stage I, 22 patients (15.7%) were stage II, 39 (27.9%) were stage III and 1 patient (0.7%) was stage IV. Comparing the surgical staging between type I and type II EAOC demonstrated that EAOC type I cases are more often of lower stage at diagnosis, 51 patients (71.8%) vs. 27 (39.1%) presented at stage I, 8 (11.3%) vs. 14 (20.3%) patients at stage II, 12 (16.9%) vs. 27 (39.1%) patients at stage III and 0 vs. 1 (1.4%) patient at stage IV, respectively, P=0.001.
Table 1 outlines p53 status and the difference in p53 expression in EAOC type I versus type II by histologic type and grade. EAOC type I is associated with histological types that are more likely to harbor p53 mutation namely high-grade serous and high grade endometrioid carcinoma. The relationship between endometriosis and ovarian cancer was not associated with P53 status when individual histological types were compared.
|
P53 Expression |
Histologic type |
EAOC type I |
EAOC type II |
|
|
''Overexpressed'' |
Endometrioid carcinoma |
4 (4)* |
3 (3)* |
|
|
Clear cell carcinoma |
1 (1)* |
0 |
||
|
Serous carcinoma |
2 (2)* |
16 (16)* |
||
|
Mucinous carcinoma |
0 |
0 |
||
|
''Null'' |
Endometrioid carcinoma |
4 (4)* |
3 (2)* |
|
|
Clear cell carcinoma |
2 (2)* |
0 |
||
|
Serous carcinoma |
3 (3)* |
10 (10)* |
||
|
Mucinous carcinoma |
0 |
1 |
||
|
''Wild-type'' pattern |
Endometrioid carcinoma |
27 (10)* |
14 (6)* |
|
|
Clear cell carcinoma |
26 (26)* |
9 (9)* |
||
|
Serous carcinoma |
2 (1)* |
8 (4)* |
||
|
Mucinous carcinoma |
0 |
4 |
||
Table 1: P53 expression by tumor grade and histology in type I and type II EAOC
Among 23 patients with null phenotype, 21 (91.3%) were high grade, 13 (56.5%) were serous carcinomas, 9 (39.1%) presented at a late stage (8 patients stages III, 1 patient stage IV) and 7 (30.4%) recurred. Among 26 patients that overexpressed p53, all carcinoma were high grade with predominantly serous histologic type (18/26, 69.2%), 12(46.1%) patients were stage III, and 10 (38.5%) recurred. Among 90 patients with p53 wild type pattern, 56 (62.2%) were high grade, with predominantly endometrioid cell type (41/90, 45.6%) followed by clear cell type (35/90, 38.9%), 19/90 (21.1%) patients presented at stage III and 17 (18.8%) recurred.
With a median follow up period of 43 months (range: 0-161), 16 (22.5%) patients had tumor recurrence among EAOC type I versus 18 (26.1%) patients with EAOC type II, P= 0.6. The median time to recurrence was 15 months (range: 3-102) in patients with EAOC type I versus 18 months (range: 1-50) in patients with EAOC type II, P=0.8. All recurrences occurred in patients with high grade carcinoma. Among patients with EAOC type I that recurred, clear cell carcinoma was the most common histologic types (10, 62.5%) followed by endometrioid carcinoma (5, 31.3%) and serous carcinoma (1, 6.3%) while the most common histologic type among recurred patients with EAOC type II was serous carcinoma (12, 66.7%) followed by endometrioid carcinoma (4, 22.2%) and clear cell carcinoma (2, 11.1%), P= 0.001.
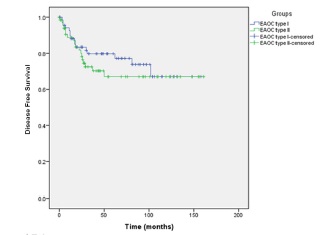
There was no significant difference between the time to recurrence, disease free survival (Figure 3) and estimated hazard ratios between the two groups. When each of the groups was further classified by p53 status, there was no significant difference between time to recurrence, the disease free survival and estimated hazard ratios function of the four subgroups. There was a significant difference in the hazard of recurrence between patients with p53 mutant and patients with wild type phenotype regardless of the EAOC type (p= 0.027).
Figure 3: Kaplan-Meier curve for disease free survival of patients with mutant p53 versus patients with wild type p53 in EAOC.
Discussion
This study compared clinical, histological and the p53 status in two subgroups of patients with EAOC based on the relationship between the cancer and endometriosis. Cases with EAOC type I differ from type II by a higher proportion of endometrioid and clear cell histologic types. The proportion of high grade endometrioid carcinoma was higher among EAOC type II. There were only 5 cases of mucinous carcinomas in the study cohort; all of them were classified as EAOC type II. Only few studies examined the potential difference between EAOC with direct transition to associated endometriosis. Garrett et al. identified 140 patients with ovarian carcinoma and investigated the outcomes of different subtypes arising in or associated with endometriosis. Among 92 patients with EAOC, 37.1% showed ovarian carcinoma with direct transition to endometriosis and 28.6% had carcinoma with endometriosis in the same ovary without direct transition. They found that carcinomas arising in or associated with endometriosis were associated with better survival than the carcinoma not associated with endometriosis. However, they did not discuss the difference between the two subtypes of endometriosis associated ovarian cancer in their abstract but patients with EAOC has improved survival that was mainly dependent on patient age, tumor stage and grade compared with patients without endometriosis [36].
In the study by Orezzoli et al. [37], 84 cases of clear cell carcinoma were identified, 49% of them has coexisting endometriosis. Only 15 of these tumors were found to arise in endometriosis while 26 cases had endometriosis that was not in contiguity with the tumor. They found that clear cell carcinomas arising in endometriosis were significantly more likely to present with early stage than those carcinoma not in contiguity with endometriosis (87% vs. 54%). They also found that patients with carcinoma arising in endometriosis were more likely to recur than those with carcinoma not in contiguity with endometriosis. The authors suggested an explanation that pelvic recurrences actually represented second pelvic primaries arising in residual endometriosis.
Sainz de la Cuesta et al. studied 22 patients diagnosed with ovarian carcinoma in the presence of endometriosis, with 7 carcinomas (32%) arising in endometriosis. In these 7 cases a spectrum of benign and atypical endometriosis with a transition to clear cell or endometrioid adenocarcinoma were identified. Most patients with endometrioid and clear cell histologist presented at stage I [38].
Modesitt et al. [39] reported on a series of 115 cases of epithelial ovarian cancers that included 25 patients with ovarian cancer arising in endometriosis and 33 patients with ovarian cancer with adjacent endometriosis. Among surgically staged patients, they found 12/20 (60%) patients versus 15/27 (55.6%) patients presented with early stage (I, II), respectively with no survival difference between the two groups. This data agrees with our data that showed that EAOC type I cases were more likely to present at a lower stage than EAOC type II with no significant difference in the disease free survival. Also support Davis et al. finding that showed no difference in the outcome of patients with carcinoma arising in endometriosis versus patients with endometriosis adjacent to carcinoma [40].
In our opinion, EAOC type II represents a mixture of cases, those that probably arose directly from endometriosis but a) the transitional zone was not demonstrated on any of the slides due to limitation of sampling or b) was ''run over'' by a more aggressive tumor (hence we see more high grade endometrioid carcinoma in this group). Other cases are probably unrelated to the presence of endometriosis, like high grade serous carcinoma which is the most common histological type of ovarian cancer and since endometriosis is a very common condition, these are likely unrelated to the presence of endometriosis.
In the current study, mutant p53 was detected in 49/139 (35.3%) of ovarian carcinoma. Of them, 16/70 (22.9%) were among carcinoma arising in endometriosis (EAOC type I) while 33/69 (47.8%) were among carcinoma associated with endometriosis (EAOC type II), P= 0.007. In the latter group, serous carcinoma was the most common histological type (26/33) that showed p53 mutation (10 carcinomas lacking the nuclear expression of p53, 16 carcinomas showed overexpression of p53), while p53 mutation was detected in 6 serous carcinomas in the former group. This reflects that p53 mutation in EAOC is mainly related to the histologic type. This finding is consistent with previous studies [28,41].
Molecular studies have shown that complete lack of p53 immunoexpression in tumors with unequivocal serous morphology harbor a p53 mutation associated with a truncated form of the protein or a protein with conformational changes that cannot be detected using commercial available antibodies [45,46]. P53 overexpression is due to missense mutation that leads to accumulation of the protein in the nucleus with strong and diffuse immunolabeling [47,48]. Null mutations are known to be related to early, distant metastasis and poor prognosis and they represent an independent predictor of poor survival in ovarian cancer [42]. However, the prognostic significance of p53 overexpression or wild-type p53 in ovarian cancer is debatable [43,44]. Yemelyanova et al. showed that immunohistochemical overexpression of p53 in more than 60% of cells or a complete loss of immunoreactivity against p53 are all indicative of mutant p53 gene, while low and focal expression of p53 (10-50%) may signify the presence of wild-type p53 gene [24].
As with all TMA based studies, one of the limitations of our study is how the tumor represented because the tissue analyzed in TMAs may not be representative of the whole specimen, especially if ovarian tumors are heterogeneous [49]. However, previous studies validated the use of tissue microarray and support its use in various epithelial tumors [50] and determined that the use of tissue micrroarrays containing one to two cores provides an adequately representative sample for analysis by IHC [51,52]. In this study, TMAs were constructed with two 1mm cores allowing for a large number of tumor samples to be studied simultaneously under the same laboratory conditions with maximum efficiency of slides numbers and time consumption. Moreover, there was nearly perfect level of concordance between both cores for most of the cases except one case with discordant results that was resolved by retesting on a full section.
Conclusion
In this study we explored whether classifying EAOC based on the ability to demonstrate direct morphologic transition adds clinical value by comparing the outcome of the two groups. To the best of our knowledge, this approach is original. In this single institution cohort, the outcome was related to the histological type and stage and the type of EAOC was not independently predictive of outcome. Moreover, biological differences such as in p53 status and stage are rather linked to the histological type and grade.
Acknowledgements
We thank the Ministry of Higher Education, Egypt which partly supported this study. From Sunnybrook Health Sciences, the authors thank: Xu Guo for her help in TMA construction, Manar Al Assi and Nagham Abdalahad for their help in slide scanning and Samira Alminawi for her help in IHC staining.
Disclosure/Declaration
The authors declare no conflict of interest.
References
- Sampson JA (1925) Endometrial carcinoma of the ovary, arising in endometrial tissue in that organ. Arch Surg 10: 1-72.
- Erzen M, Rakar S, Klancnik B, Syrjänen K (2001) Endometriosis-associated ovarian carcinoma [EAOC]: an entity distinct from other ovarian carcinomas as suggested by a nested case-control study. Gynecol Oncol 83: 100-108.
- Melin A, Lundholm C, Malki N, Swahn ML, Sparen P, et al. Endometriosis as a prognostic factor for cancer survival. Int J Cancer 129: 948-955.
- McMeekin DS, Burger RA, Manetta A, DiSaia P, Berman ML (1995) Endometrioid adenocarcinoma of the ovary and its relationship to endometriosis. Gynecol Oncol 59: 81-86.
- Komiyama S, Aoki D, Tominaga E, Susumu N, Udagawa Y, et al. (1999) Prognosis of Japanese patients with ovarian clear cell carcinoma associated with pelvic endometriosis: clinicopathologic evaluation. Gynecol Oncol 72: 342-346.
- Orezzoli JP, Russell AH, Oliva E, Del Carmen MG, Eichhorn J, et al. (2008) Prognostic implication of endometriosis in clear cell carcinoma of the ovary. Gynecol Oncol 110: 336-344.
- Kumar S, Munkarah A, Arabi H, Bandyopadhyay S, Semaan A, et al. (2011) Prognostic analysis of ovarian cancer associated with endometriosis. Am J Obstet Gynecol 204: 63.e1-7.
- Cuff J, Longacre TA (2012) Endometriosis does not confer improved prognosis in ovarian carcinoma of uniform cell type. Am J Surg Pathol 36: 688-695.
- Katagiri A, Nakayama K, Rahman MT, Rahman M, Katagiri H, et al. (2012) Loss of ARID1A expression is related to shorter progression-free survival and chemoresistance in ovarian clear cell carcinoma. Mod Pathol 25: 282-288.
- Sampson J (1927) Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Obstet Gynecol 14: 422-469.
- Scott R (1953) Malignant change in endometriosis. Obstet Gynecol 2: 283-289.
- Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, et al. (2000) Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 88: 2584-2589.
- Oral E, Ilvan S, Tustas E, Korbeyli B, Bese T, et al. (2003) Prevalence of endometriosis in malignant epithelial ovary tumours. Eur J Obstet Gynecol Reprod Biol 109: 97-101.
- Valenzuela P, Ramos P, Redondo S, Cabrera Y, Alvarez I, et al. (2007) Endometrioid adenocarcinoma of the ovary and endometriosis. Eur J Obstet Gynecol Reprod Biol 134: 83-86.
- Boyraz G, Selcuk I, Yaz?c?o?lu A, Tuncer ZS (2013) Ovarian carcinoma associated with endometriosis. Eur J Obstet Gynecol Reprod Biol 170: 211-213.
- Lamb P, Crawford L (1986) Characterization of the human p53 gene. Mol Cell Biol 6: 1379-1385.
- Prives C, Hall AP (1999) The p53 pathway. J Pathol 187: 112-126.
- Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW (1991) Participation of p53 protein in the cellular response to DNA damage. Cancer Res 51: 6304-6311.
- Kraiss S, Spiess S, Reihsaus E, Montenarh M (1991) Correlation of metabolic stability and altered quaternary structure of oncoprotein p53 with cell transformation. Exp Cell Res 192: 157-164.
- Greenblatt MS, Bennett WP, Hollstein M, Harris CC (1994) Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res 54: 4855-4878.
- Psyrri A, Kountourakis P, Yu Z, Papadimitriou C, Markakis S, et al. (2007) Analysis of p53 protein expression levels on ovarian cancer tissue microarray using automated quantitative analysis elucidates prognostic patient subsets. Ann Oncol 18: 709-715.
- Rogel A, Popliker M, Webb CG, Oren M (1985) p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol 5: 2851-2855.
- Hall PA, Lane DP (1994) p53 in tumour pathology: can we trust immunohistochemistry?--Revisited! J Pathol 172: 1-4.
- Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, et al. (2011) Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol 24: 1248-53.
- Hartmann LC, Podratz KC, Keeney GL, Kamel NA, Edmonson JH, et al. (1994) Prognostic significance of p53 immunostaining in epithelial ovarian cancer. J Clin Oncol 12: 64-69.
- Baekelandt M, Kristensen GB, Nesland JM, Tropé CG, Holm R (1999) Clinical significance of apoptosis-related factors p53, Mdm2, and Bcl-2 in advanced ovarian cancer. J Clin Oncol 17: 2061.
- Khalifa MA, Lacher DA, Lage JM, Mannel RS, Walker JL, et al. (1997) Immunohistochemical assessment of proliferation markers and altered gene expression in archival specimens of ovarian epithelial tumors. Cancer Detect Prev 21: 532-539
- Kurman RJ, Shih IeM (2008) Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol 27: 151-160.
- Köbel M, Kalloger SE, Carrick J, Huntsman D, Asad H, et al. (2009) A limited panel of immunomarkers can reliably distinguish between clear cell and high-grade serous carcinoma of the ovary. Am J Surg Pathol 33: 14-21.
- Köbel M, Bak J, Bertelsen BI, Carpen O, Grove A, et al. (2014) Ovarian carcinoma histotype determination is highly reproducible, and is improved through the use of immunohistochemistry. Histopathology 64:1004-1013.
- Movahedi-Lankarani S, Baker PM, Giks B, Soslow RA, Otis CN, et al. (2013) Protocol for the Examination of Specimens From Patients With Carcinoma of the Ovary. College of American Pathologists.
- Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17: 1471-1474.
- Kato N, Sasou S, Motoyama T (2006) Expression of hepatocyte nuclear factor-1beta (HNF-1beta) in clear cell tumors and endometriosis of the ovary. Mod Pathol 19: 83-89
- Nafisi H, Ghorab Z, Ismill N, Dubé V, Plotkin A, et al. (2015) Immunophenotypic Analysis in Early Müllerian Serous Carcinogenesis. Int J Gynecol Pathol 34: 424-436.
- Silverberg SG (2000) Pathologic grading of ovarian carcinoma: a review and proposal. Int J Gynecol Pathol 19: 7-15.
- Garrett LA, Growdon WB, Goodman A, Boruta DM, Schorge JO, et al. (2013) Endometriosis-associated ovarian malignancy: a retrospective analysis of presentation, treatment, and outcome. J Reprod Med 58: 469-476.
- Orezzoli JP, Russell AH, Oliva E, Del Carmen MG, Eichhorn J, et al. (2008) Prognostic implication of endometriosis in clear cell carcinoma of the ovary. Gynecol Oncol 110: 336-344.
- Sainz de la Cuesta R, Eichhorn JH, Rice LW, Fuller AF Jr, Nikrui N, et al. (1996) Histologic transformation of benign endometriosis to early epithelial ovarian cancer. Gynecol Oncol 60: 238-244.
- Modesitt SC, Tortolero-Luna G, Robinson JB, Gershenson DM, Wolf JK (2002) Ovarian and extraovarian endometriosis-associated cancer. Obstet Gynecol 100: 788-795.
- Davis M, Rauh-Hain JA, Andrade C, Boruta DM, Schorge JO, et al. (2014) Comparison of clinical outcomes of patients with clear cell and endometrioid ovarian cancer associated with endometriosis to papillary serous carcinoma of the ovary. Gynecol Oncol 132: 760-766.
- Klemi PJ, Pylkkänen L, Kiilholma P, Kurvinen K, Joensuu H (1995) p53 protein detected by immunohistochemistry as a prognostic factor in patients with epithelial ovarian carcinoma. Cancer 76: 1201-1208.
- Tashiro H, Isacson C, Levine R, Kurman RJ, Cho KR, et al. (1997) p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol 150: 177-1785.
- Kumar V, Abbas AK, Fausto N, Aster J (2010) Robbins & Cotran Pathologic Basis of Disease. (8th edtn), Saunders.
- Bennett WP, Hollstein MC, Hsu IC, Sidransky D, Lane DP, et al. (1992) Mutational spectra and immunohistochemical analyses of p53 in human cancers. Chest 101(3 Suppl): 19S-20S.
- Bartek J, Iggo R, Gannon J, Lane DP (1990) Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene 5: 893-899.
- Shahin MS, Hughes JH, Sood AK, Buller RE (2000) The prognostic significance of p53 tumor suppressor gene alterations in ovarian carcinoma. Cancer 89: 2006-2017.
- Niwa K, Itoh M, Murase T, Morishita S, Itoh N, et al. (1994) Alteration of p53 gene in ovarian carcinoma: clinicopathological correlation and prognostic significance. Br J Cancer 70: 1191-1197.
- Fallows S, Price J, Atkinson RJ, Johnston PG, Hickey I (2001) p53 mutation does not affect prognosis in ovarian epithelial malignancies. J Pathol 194: 68-75.
- Reles A, Wen WH, Schmider A, Gee C, Runnebaum IB (2001) Correlation of p53 mutations with resistance to platinum-based chemotherapy and shortened survival in ovarian cancer. Clin Cancer Res 7: 2984-2997.
- Kuwabara S, Ajioda Y, Watanabe H, Hitomi J, Nishikura K, et al. (1998) Heterogeneity of p53 mutational status in esophageal squamous cell carcinoma. Jpn J Canc Res 89: 405-410.
- Rimm DL, Camp RL, Charette LA, Costa J, Olsen DA, et al. (2001) Tissue microarray: a new technology for amplification of tissue resources. Cancer J 7: 24-31.
- Camp RL, Charette LA, Rimm DL (2000) Validation of tissue microarray technology in breast carcinoma. Lab Invest 80: 1943-1949.