Research Article
Jay K Udani, Pee-Win Chong, Emily Teng*, and Joerg Gruenwald
*InQpharm Group Europe Ltd, Malaysia
Corresponding author
Emily Teng, InQpharm Group Europe Ltd, E-16 Plaza Mont Kiara, 2 Jalan Kiara, 50480 Kuala Lumpur, Malaysia, Tel: +60 3 6201 9690; E-mail: eteng@inqpharm.com
Received Date: 26th October 2015
Accepted Date: 07th December 2015
Published Date: 10th December 2015
Citation
Udani JK, Chong PW, Teng E, Gruenwald J (2015) Effect of Litramine (IQP-G-002AS) on Weight Loss in Overweight and Obese US Population: A Randomised, Double-Blind, Placebo-Controlled Study. Enliven: J Diet Res Nutr 2(3): 006.
Copyright
@ 2015 Emily Teng. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Previous studies have shown that Litramine was effective in weight loss for overweight and obese subjects.
Objective:
To further validate the weight loss effect of Litramine compared to placebo in US population.
Methods:
Double-blind, randomised, placebo-controlled study conducted over 14 weeks including 2 weeks run-in. Overweight and obese subjects were randomly
allocated to either Litramine™ or the placebo group. Primary endpoint was the comparison of the difference in body weight (lbs) at baseline and after 12
weeks of treatment with Litramine™ versus placebo.
Results:
The Litramine group lost significantly more weight in comparison to the placebo group who gained weight instead (-2.53 lbs vs 2.56 lbs, p<0.001).
Conclusion:
Litramine is safe and efficacious for body weight loss.
Keywords
Litramine; Weight loss; IQP-G-002AS
Abbeviations
lbs: Pounds; BMI: Body Mass Index
Introduction
Since 1980, worldwide obesity has more than doubled with an estimated 10% of world population classified as obese [1]. It is projected that by 2048, all American adults will become overweight or obese based on 2012 trends [1]. In 2010, overweight and obesity were estimated to cause 3.4 million deaths worldwide [2]. This epidemic has also placed higher burdens on healthcare systems, as those who are obese are at higher risk for many serious health conditions such as cardiovascular diseases, type 2 diabetes and certain cancers [3-5].
Weight loss reduces morbidity risk in metabolic diseases. The INTERHEART study found that a sustained weight loss of just 5-10% can have a positive impact on metabolic and cardiovascular risks [6]. The same amount of weight loss can also reduced progression of diabetes and improve diabetes control [7,8]. Body weight can be influenced by complex factors such as genetics and metabolism but one of the main contributary factor is positive energy balance. According to a recent analysis, increased food energy supply has been implicated as a major driver of this global epidemic [9]. Treatments that aim to reduce caloric intake and absorption of dietary fat can be considered as effective approaches to reducing weight [10].
The investigational product, Litramine (IQP-G-002AS) is a patented natural fibre complex derived from Opuntia ficus-indica, that has been enriched with additional soluble fibre from Acacia sp. Litramine has been standardised for its lipophilic activity, where 1g of Litramine binds to 10g of fats. In the gut, Litramine forms fat-fibre complexes which are indigestible thus reduces calories uptake. Its mechanism of action has been largely described in other studies [11,12]. There have been two previous studies which confirmed that Litramine is safe and efficacious for weight loss and weight maintenance [12,13]. This study was conducted to further validate the efficacy of Litramine as a weight loss supplement under a different study set up of a US population.
Method and Procedure
Trial Design
This double-blind, randomised, placebo-controlled study was conducted over 14 weeks including a 2-week run-in period at a study center in California, USA. Subjects were randomly allocated in 1:1 ratio to either the Litramine or the placebo group. All subjects gave written informed consent voluntarily. The clinical investigation was approved by the Western Institutional Review Board® (Olympia WA), and was performed in compliance with EN ISO 14155, the Declaration of Helsinki, and the Guideline for Good Clinical Practice (CPMP/ICH/135/95).
Eligible subjects included overweight and obese subjects (BMI 25-35 kg/m2) between the ages of 18 to 60 years who are accustomed to three main meals per day, with a consistent and stable body weight 3 months prior to study enrolment. Women of childbearing age were required to use appropriate birth control methods during the duration of study. Subjects who had known sensitivity to the ingredients in the investigational product were excluded from the study. Other exclusion criteria included subjects with a history of medical disorders that may affect body weight (such as diabetes mellitus, Cushing?s disease etc.), presence of any active gastrointestinal (GI) disease or malabsorption disorders, history of GI surgery, history of eating disorders, history of cardiac disease or renal disease, pregnant and lactating women and subjects on medication that could influence GI function. Subjects using other anti-obesity products are excluded unless such medications were discontinued 3 months prior to the study initiation. Subjects who were taking daily dietary supplements were allowed to participate, provided that they stop taking the supplements 2 weeks before the start of the study. Subjects were also excluded if they were currently on any diet regimen (such as low carbohydrate, low fat or high protein diets), or in case they undergo more than 3 hours of strenous physical activity per week.
At screening visit, subjects first provided their informed consent. Inclusion and exclusion criteria were then assessed. A detailed medical history was obtained from each of the subjects and a physical examination was conducted by the investigator. Body weight and blood pressure measurement were also taken. Subjects then underwent a two-week washout period of any previous dietary supplement intake, and kept a diary on food intake and satiety. Subjects who are compliant (>85%) in completing the diary were included.
Eligible subjects received either two tablets (1g) of Litramine or matching placebo, to be taken three times a day after each main meal. The placebo tablet was identical in appearance to the Litramine tablet but contained 500 mg of microcrystalline cellulose as replacement for the active ingredient. Tablets were packed into bottles and labeled by an independent pharmacist for each subject according to the randomisation schedule. Both the investigators and the subjects were not informed of the treatment allocation throughout the study.
Subjects were followed up through scheduled visits with the investigators on week 4, 8 and 12. At each visit, subjects were required to fast for 10 hours prior to the visit. This was to minimise influence from recent food consumption on measurements.
All subjects were counselled after each study visit to maintain a nutritionally balanced diet according to USDA recommendation. However no formal dietary restriction or any behavioral modification programmes were applied to simulate ?free-living conditions?. The subjects were asked to keep a diary documenting their daily intake of the investigational product and also their food intake. Subjects were also asked to complete the Stanford Exercise Behavious scale [14], which is used to determine the level of exercise that the subject had undertaken.
End Points
The primary endpoint of this study was the comparison of the the difference in body weight (lbs) at baseline and after 12 weeks of treatment with Litramine? versus placebo in overweight and obese subjects. Body weight was measured using a calibrated weighing scale in subjects wearing underwear and no shoes at screening, baseline and subsequently at 4-week intervals.
Secondary endpoints included changes in mean waist circumferences, body mass index (BMI) and mean body fat mass. Waist circumference (inches) was measured at the level midway between the lateral lower rib marin and the iliac crest. Body fat content was measured by bioimpedence method using validated electronic weighing scales.
Safety Parameters
Safety assessment included measurement of vital signs such as resting blood pressure, heart rate (beats per minute), body temperature (°F) and respiratory rate (breaths per minute). The subjects were asked open-ended questions to establish any adverse effects at each visit. All adverse effects were recorded regardless of causality.
Statistical Analyses
Demographic and baseline characteristics of efficacy and safety variables were assessed based on descriptive statistics. All primary and secondary variables, as well as safety variables were evaluated as relative change within the groups. For all statistical analyses, a level of significance of p<0.05 was assumed. All values are presented as mean (SD) unless indicated otherwise. Efficacy and safety data were analyzed based on modified per protocol data, which was defined as subjects who had at least one post-dose visit completed. All statistical analyses was done using the SPSS Statistic software, V19.0 (SPSS, Chicago, IL).
Results
A total of 217 subjects were screened with 99 subjects being randomised into two groups. 3 subjects were excluded after randomisation due to non-compliance. 9 subjects in the Litramine group and 10 subjects in the placebo group did not turn up for the 2nd follow up visit and were thus excluded from analysis. All remaining subjects attended all clinic visits as planned. The baseline characteristics are shown in Table 1 below. There were no significant differences between both groups.
|
Parameter |
Litramine (n=48) |
Placebo (n=48) |
|
Gender |
|
|
|
Male |
15 |
16 |
|
Female |
33 |
32 |
|
Age (years) |
38.33 (11.38) |
39.63 (11.19) |
|
Weight (lbs) |
186.93 (33.26) |
185.28 (22.37) |
|
Height (inches) |
65.50 (3.88) |
66.12 (3.56) |
|
BMI (kg/m2) |
30.45 (3.38) |
29.76 (2.47) |
Table 1: Baseline characteristics of randomised subjects. Values are presented as mean (SD).
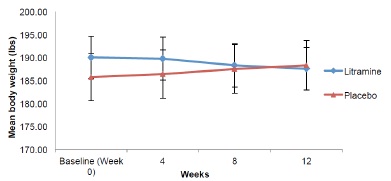
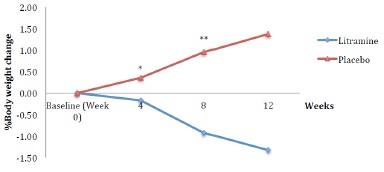
Figure 1 shows mean body weight of the subjects throughout the study. The mean body weights of the subjects at baseline were 190.06 (SD 28.66) lbs in the Litramine group and 185.74 (SD 31.91) lbs in the placebo group. There is a trend towards weight reduction in the Litramine group compared to the placebo group. At the end of week 12, mean body weight in the Litramine group was 187.53 (SD 28.87) lbs vs 188.30 (SD 33.16) lbs in the placebo group. The Litramine group lost significantly more weight than the placebo group compared to the placebo group who gained weight instead (Table 2). In terms of percentages, those taking Litramine actually lost 1.33% of their initial body weight compared to those on placebo who gained 1.38% instead (Figure 2).
Figure 1: Body weight reduction over time (Error bars represent SEM)
Figure 2: % Body weight reduction over time. *p=0.035, **p<0.000
|
Parameter |
Litramine (n=39) |
Placebo (n=38) |
p-value |
|
Body weight (lbs) |
-2.53 (3.75) |
2.56 (4.73) |
<0.001 |
|
BMI (kg/m2) |
-0.41 (0.60) |
0.43 (0.75) |
<0.001 |
|
Waist circumference |
-1.56 (5.98) |
0.55 (6.75) |
0.213 |
|
Body fat (%) |
-0.18 (6.71) |
-0.44 (4.56) |
0.836 |
Table 2: Mean changes in primary and secondary parameters between baseline and week 12 (Negative values represent reduction). Values are presented as mean (SD)
BMI
Subjects in the Litramine group had a mean BMI of 30.35 (SD 3.22) kg/m2 compared to the placebo group at 30.51 (SD 3.28) kg/m2 at baseline. At the end of the study, there was a significant reduction in BMI for subjects in the Litramine at -0.41 (SD 0.60) kg/m2 compared to the subjects in the placebo group whose BMI gained slightly at 0.43 (SD 0.75) kg/m2 (Table 2) compared to baseline.
Waist Circumference
At baseline, subjects in the Litramine group had a mean waist circumference of 39.40 (SD 4.74) inches while the placebo group had a mean waist circumference of 38.63 (SD 4.16) inches. Although the change in waist circumference measured at the end of the study was not statistically significant between the Litramine and placebo groups, the Litramine group showed a trend towards reduction in waist circumference, whereas the placebo showed an increasing trend.
Body Fat Percentage
After 12 weeks, there was a slight decrease for both Litramine and placebo group, which was not statistically significant. Subjects in Litramine group had their body fat percentage drop by 0.18 (SD 6.71)% while those in placebo group reduced by 0.44 (SD 4.56)%.
Safety and Tolerability
There were no clinically significant changes in mean body temperature, mean blood pressure and mean heart rate during the study. Throughout the study, there were 18 reported cases of adverse events in 12 subjects. Reported cases included upper respiratory tract infections and other common cold symptoms, which were assessed by the investigator not to be related to intake of the study product. There was one case of allergic rash, which was possibly linked to intake of the study product. This reaction was deemed not serious by the investigators and a decision was made to continue with the study. The condition was then resolved after a month. No serious adverse events were reported during the course of the study.
Discussion
In this study, we looked at the use of Litramine as a weight loss aid in conjunction with a balance diet. EU and US guidelines recommend that those who have a BMI > 25 kg/m2 should first be counselled on lifestyle changes and nutritional modifications, through reduction of caloric intake, before consideration of other methods.
Litramine works via mechanical and physical means within the gastrointestinal tract. Previous studies have shown Litramine to be effective in weight loss through the binding of dietary fat [12,13]. The fat-fibre complexes formed are not absorbable and prevent further utilisation or storage of the dietary fat in the body, resulting in a lower caloric uptake.
In the current study, there were significant changes in subjects? BMI and weight. Although the weight reduction was short of the 5% weight loss stipulated for significant health risk reduction, it must be borne in mind that this study was conducted with the purpose to investigate the effectiveness of Litramine in a population not instructed to modify their diet or lifestyle. Therefore, this study results reasonably support the conclusion that Litramine is effective in weight loss even if those who are taking it do not apply any stringent behavioural modifications. However, we recommend the intake of the product in combination with a slightly hypocaloric dietarian regime, similar to the conditions applied in the previous clinical trial to achieve a better weight loss effect [12].
Current marketed antiobesity drugs often cause abdominal discomfort and are associated with liver injury, cardiac and psychiatric side effects [15-17]. The lipase inhibitor, Orlistat, which is commonly prescribed and available over the counter to help with weight loss, is reported to cause unpleasant side effects such as oil spotting [15]. In comparison, Litramine is a safe and effective alternative to aid in weight loss. No serious adverse effects were reported during the course of this study. The active in Litramine has long been used as a food source and its safety has also been shown in previous clinical trials [12,13].
Limitations
There was no follow-up performed after termination of the study, and subsequent body weight regain post-study may occur due to the well known ?yo-yo? effect in conjunction with dietary interventions. However a previous weight maintenance study conducted with Litramine showed that continuation of the intake over a period of 24 weeks does also support weight maintenance [13].
The study duration was relatively short. Besides measuring weight loss, it was not able to measure effect of Litramine in improving obesity-related health risks.
Conclusion
Evidence from previous studies showed that Litramine is an efficacious mean towards weight reduction. This study reiterates the fact that Litramine can be considered as an effective weight loss tool in combination with a healthy lifestyle.
References
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. (2014) Global, regional and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 766-781.
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, et al. (2012) A Compartive risk assessment of burden of disease and injury attributable to 67 risks factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the GLobal Burden of Disease Study 2010. Lancet 380: 2224-2260.
- World Health Organisation (2015) Fact sheet no.311: Obesity and Overweight.
- Haslam DW, James WP (2005) Obesity. Lancet 366: 1197-1209.
- Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, et al. (2015) Global burden of cancer attributable to high body-mass index in 2012: a population based study. Lancet Oncol 16: 36-46.
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, et al. (2004) INTERHEART study investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364: 937-952.
- Wing RR, Venditti E, Jakicic JM, Polley BA, Lang W (1998) Lifestyle intervention in overweight individuals with a family history of diabetes. Diabetes Care 21: 350-359.
- Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, et al. (1987) Long-term effects of modest weight loss in Type II diabetic patients. Arch Intern Med 147: 1749-1753.
- Vandevijvere S, Chow CC, Hall KD, Umali E, Swinburn BA (2015) Increased food energy supply as a major driver of the obesity epidemic: a global analysis. Bull World Health Organ 93: 446-456.
- Tsigos C, Hainer V, Basdevant A, Finer N, Fried M, et al. (2008) Management of obesity in adults: European clinical practice guidelines. Obes Facts 1: 106-116.
- Uebelheck R, Busch R, Alt F, Beah ZM, Chong PW (2014) Effects of cactus fiber on the excretion of dietary fat in health subjects: A double blind, randomised, placebo-controlled, crossover clinical investigation. Curr Ther Res Clin Exp 76: 39-44.
- Grube B, Chong PW, Lau K, Orzechowski H (2013) A natural fibre complex reduces body weight in the overweight and obese: a double blind, randomised, placebo-controlled study. Obesity (Silver Spring) 21: 58-64.
- Grube B, Chong PW, Alt F, Uebelhack R (2015) Weight maintenance with Litramine (IQP-G-002AS): A 24-week double-blink, randomised, placebo-controlled study. J Obes 953138.
- Stanford Chronic Disease Self-Management Study, Psychometrics, Lorig K, Stewart A, Ritter P, Gonzalez V, Laurent D, Lynch J (1996) Outcome Measures for Health Education and other Health Care Interventions. CA: Sage Publications 25: 37-38.
- Johansson K, Neovius K, DeSantis SM, Rössner S, Neovius M (2009) Discontinuation due to adverse events in randomised trials of orlistat, sibutramine and rimonabant: a meta-analysis. Obes Rev 10: 564-575.
- Cheung BM, Cheung TT, Samaranayake NR (2013) Safety of antiobesity drugs. Ther Adv Drug Saf 4: 171-181.