Research Article
Authors: Bindu Gandrapu MD1, and David E. Henner DO2*
1 Division of Nephrology, Berkshire Medical Center, Pittsfield, USA
2 Medical Director of Dialysis Unit, Chief, Division of Nephrology, Berkshire Medical Center, Pittsfield, USA
Corresponding author
David E. Henner DO, Medical Director of Dialysis, Division Chief of Nephrology, Berkshire Medical Center, 777 North Street, Pittsfield, MA 01201 USA
Tel: (413) 447-2000
E-mail: dhenner@bhs1.org;
Received Date: 18th July 2017;
Accepted Date: 1st September 2017;
Published Date: 10th September 2017
Citation
Gandrapu B, Henner DE (2017) Does Ultrapure Dialysis Fluid improve Quality of Life in Hemodialysis Patients?. Enliven: Nephrol Renal Stud 4(1): 002.
Copyright
@ 2017 David E. Henner DO. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited..
Abstract
Introduction: Use of ultrapure dialysis fluid (UPDF) may offer hemodialysis patients advantages over conventional dialysate, including reduced infection risk, preserved residual kidney function, improved nutrition, reduced inflammation and improved anemia. In 2011, a new standard was published which defined differences between standard dialysis fluid and ultrapure dialysis fluid. It’s unclear if these advantages will be seen if UPDF is widely adopted.
Methods: In 2013, our dialysis facility at Berkshire Medical Center, Pittsfield, Massachusetts installed a new water treatment system capable of producing ultrapure water for dialysis fluid. We hypothesized that use of UPDF might lead to improved quality of life, as measured by Kidney Disease and Quality of Life (KDQOL-36) scores. We examined the available KDQOL-36 scores of patients up to 18 months prior to and after installation of the new water System. We analyzed KDQOL-36 scores for these patients in 5 subscales, and calculated the mean change of each score, and analyzed the data for differences in the groups prior to and after installation of ultrapure water system.
Results: We were unable to demonstrate a difference in KDQO-36 scores in any of the 5 subscales from prior to and after installation of the water system. Values for comparison of KDQOL-36 scores pre-installation of new water system vs post installation for each subscale were as follows: Effects of Kidney Disease on Daily Life p value 0.682, Physical Component Summary (PCS) p value 0.775; Mental Component Summary (MCS) p value 0.464; Burden of Kidney Disease p value 0.791, and Symptoms p value 0.862
Conclusion: According to our study, it is unclear whether the use of ultrapure dialysate will improve the quality of life in dialysis patients. However, given all the potential benefits of ultrapure dialysis fluid, use of ultrapure dialysis fluid compared to the standard dialysate fluid still deserves further study and should still be considered for use in the dialysis facilities
Keywords Ultrapure dialysis fluid (UPDF); Hemodialysis patients; Kidney disease and quality of life (KDQOL-36) score; Quality of life of patients; Association for the advancement of medical instrumentation (AAMI)
Introduction
Patients undergoing hemodialysis are exposed to very large volumes of water in the dialysis fluid used for their treatments. During an average 4-hour dialysis treatment with a typical dialysate flow of 500 ml/min, the patient may be exposed to 120 liters of water. The semipermeable membrane contained in the dialyzer acts as the only protection of the patient’s blood against any impurities or microbial pathogens contained in the pure water, that were not removed by the water treatment system. In addition, even if the water has no impurities, there may be contamination in the bicarbonate concentrate which mixes with the water and acid concentrate to form the final dialysis solution [1]. In the United States, the Center for Medicare and Medicaid Services (CMS) in the Conditions for Coverage for End Stage Renal Disease facilities, adopted the standards for pure dialysis fluid and water contained in the Association for the Advancement of Medical Instrumentation’s (AAMI) RD52 standards. Therefore, most dialysis centers in the United States currently follow these standards of pure dialysate. However, since these AAMI standards were 1st published in 2004, AAMI has updated their standards for dialysate purity several times in conjunction with the International Organization for Standardization (ISO) in 2011, AAMI released a new set of Standards “ANSI/AAMI/ISO 23500, Guidance for the Preparation and Quality Management of Fluids for Hemodialysis and Related Therapies”. In these standards, AAMI recommended the use of ultrapure dialysis fluid in dialysis fluid preparation and stated that standard dialysis fluid is but a minimum standard [2-4]. However, despite this recommendation by AAMI, standard dialysis fluid continues to be used by most dialysis centers in the United States. UPDF may offer a significant advantage over conventional dialysis fluid including decreased risk of infections [5], better preservation of residual kidney function, improved nutritional status [6-9], a higher seroconversion rate among the patients who receive intramuscular hepatitis B vaccination [7], decrease in markers of inflammation and oxidative stress, and reduction in the demand of erythropoietin, which has been shown to improve anemia management and lead to less erythropoietin stimulating agents (ESAs) use in dialysis patients [10]. These recommendations by AAMI are mainly based on observational data so it isn’t clear if these recommendations will prove to be true if widely adopted by dialysis centers in the United States.
Our dialysis unit and at Berkshire Medical Center, Pittsfield, Massachusetts installed a new water treatment system (see figure 1) capable of producing ultrapure water for dialysis fluid in February 2013. Our study examined the possible effects of ultrapure dialysis fluid on the quality of life of our dialysis patients, as measured by the standard Kidney disease and Quality of Life (KDQOL-36) survey. The KDQOL-36 survey was chosen as the preferred “standardized mental and physical assessment tool” by CMS in the Conditions for Coverage for ESRD facilities and the accompanying interpretive guidance. CMS Mandated in the Interpretative Guidance that a standardized tool such as the KDQOL-36 be performed on every dialysis patient in the United States after 3 months of being on dialysis and then annually there after.
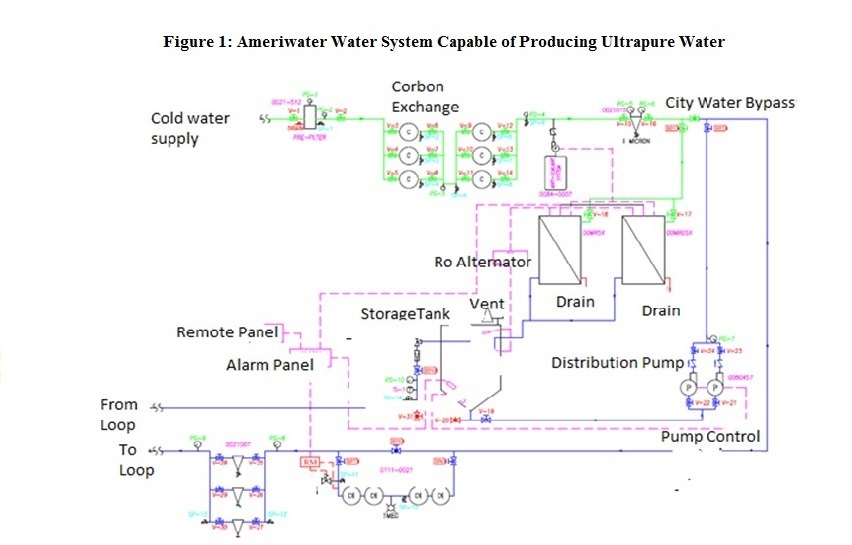
Figure1: Ameriwater Water System Capable of Producing Ultrapure Water
We hypothesized that use of UPDF might lead to the improved quality of life of patients on hemodialysis, compared to patients dialyzed with conventional dialysate fluid, due to reduced inflammation, infections, and the other advantages described above. We examined the KDQOL-36 scores of 48 patients retrospectively, comparing their KDQOL scores prior to and after implantation of our newly installed water treatment system capable of producing ultrapure water and dialysis fluid during the period between 08/10/2011 -09/10/2014.
Methods
This case series was conducted at Berkshire Medical Center, in Pittsfield Massachusetts, which is located in rural Berkshire County, in Western Massachusetts. The case series was conducted by reviewing the data from KDQOL-36 subscale scores of the 48 dialysis patients in our center on each patient both prior to and after we implemented our new water system capable of producing ultrapure water. The patients that were included in the study were those on in-center hemodialys is at least 3x/week in whom there were KDQOL-36 subscale scores available within 18 months prior to and after implementation of our new water system during the period of August, 2011 through September 2014. Of the 48 patients, 18 were female, and 30 were male. The age distribution of patients in the study ranged from 25 years old up to 91 years old with a mean age of 68 years old. Females in the study ranged in age from 25 to 89 years old, with a mean age of 67 years old. Males in the study age ranged from 39 to 91 years old, with a mean age of 69 years old. 28 of the 48 patients in this case series were diabetic. 7 patients in the case series were African American, 1 was Latino, and 40 were Caucasian.
The KDQOL-36 survey is a kidney disease-specific measure of health related quality of life (HRQOL). The KDQOL-36 is recommended by CMS and considered reliable and valid. It contains 36 items with 5 subscales. Questions 1-12 make up the SF-12 (a shorter version of the original SF-36), and are incorporated in the Physical Component Summary (PCS) subscale, and Mental Component Summary (MCS) subscale. Questions 13-28 contain kidney disease specific questions. Questions 13-16 are included in the Burden of Kidney Disease (Burden) subscale. Questions 17-28 are included in Symptoms and Problems (Symptoms) subscale, and questions 29-36 in Effects of Kidney Disease on Daily Life (Effects) subscale [11].
We utilized the KDQOL-36 Complete software to analyze the results of the survey of each patient, and score each patient in the 5 subscales in the KDQOL-36 survey as described above, including PCS, MCS, Burden, Symptoms and Effects. Developed by the non profit organization, Medical Education Institute [MEI], the KDQOL-36 Complete software utilizes ascoring system for the surveys that was developed by RAND, and compares the scores to data from Dialysis Outcomes and Practice Patterns Study (DOPPS). KDQOL-Complete scores for each subscale are case-mix adjusted for age, gender, and diabetes status. It reports individual patient subscale scores by tertiles: Above average (more than one standard deviation above the mean), Average (mean ± one standard deviation), and below average (more than one standard deviation below the mean [11,12].
The Kidney Disease and Quality of Life (KDQOL-36) survey was offered to all eligible patients on hemodialysis, in accordance with the CMS Interpretive Guidance, as part of their comprehensive interdisciplinary assessment (CIA) 3 months after starting dialysis, or their annual CIA. As part of our standard practice, eligible patients who agreed to answer the KDQOL-36 survey completed the survey either self-administered, or if they needed assistance, administered by social worker. Patient was given information about the survey prior to administration, and informed consent was obtained from each patient. Patient confidentiality was ensured. During the study period between 08/10/2011-09/10/2014, only 48 of our hemodialysis patients had KDQOL scores prior to, and after the implantation of our newly installed water treatment system, and therefore were included in this study. Patients whose KDQOL scores were not available prior to the implementation of our ultrapure water system were excluded from the study.
Using Microsoft Excel 2010, we analyzed the scores of each patient from the KDQOL-36 Complete software in all 5 subscales prior to and after the installation of our ultrapure water system in our dialysis center, and then calculated the mean change of each score. The data was then analyzed for differences in the groups prior to and after installation of ultrapure water system using both 2 Sample T tests and 2 variance tests in Minitab 16.
Ultra-Purification of Dialysis Fluid at our Dialysis Unit at Berkshire Medical Center
The standard dialysis water treatment system capable of producing pure water at BMC was replaced in February 2013 by a water system manufactured by Ameriwater, which was 510K certified by the United States Food and Drug Administration (FDA), and capable of producing ultrapure water (see Figure1). The system includes typical components of a dialysis water treatment system for pre treatment of water including a backflow preventer, blend valve, booster pump, cartridge sediment filter, deescalate (PT-401 antiscalant/scale inhibitor), total of 6 carbon tanks capable of achieving an empty bed contact time (EBCT) of 10 minutes, and a 1 micron Reverse osmos is Prefilter. 2 Reverse Osmosis RO membrane machines (MRO50) are utilized in Alternating manner to produce pure water, typically with a conductivity measured at 2 µS, which is stored in a 250-gallon storage tank. The water system then contains 4 Deionozation (DI) tanks in line in the water loop following the storage tank which typically produce water with a resistivity of 17.8-17.9 mgohm. Next in line in the water loop are 3 endotoxin grade filters before the water loop exits the water treatment room. Another endotoxin grade filter (Nephros) is contained at each dialysis station prior to the water lines which enter each dialysis machine (Gambro Phoenix) which are used for hemodialysis. Each dialysis machine also contains a Diaclear filter to reduce risk of contamination of dialysis fluid prior to the dialyzer of microbes or endotoxins.
Results
A total of 48 patients (18 females and 30 males) aged 25-91 years old (mean age 68 years old) undergoing dialysis three times a week were included on the study. Females included in the study ranged from 25 to 89 years old (mean age 67 years old,) and males ranged from 39 to 91 years old (mean age 69 years). Of the 48 patients included in the study, 28 were diabetic. 40 patients were Caucasian, 7 were African American and one was Latino. Since each patient in this case series had individual KDQOL subscales measured prior to and after installation of the Ultrapure water system, there is no difference between the groups measured before and after the installation of the water system (each group contains the same patients).
Individual p values for pre versus post scores were as follows: Effects 0.682; PCS 0.775; MCS 0.464; Burden 0.791; and Symptoms 0.862. These values indicate that there was no difference in the KDQOL scores in any of the 5 subscales from prior to and after installation of the ultrapure water system (see figures 2,3,4,5 and 6)
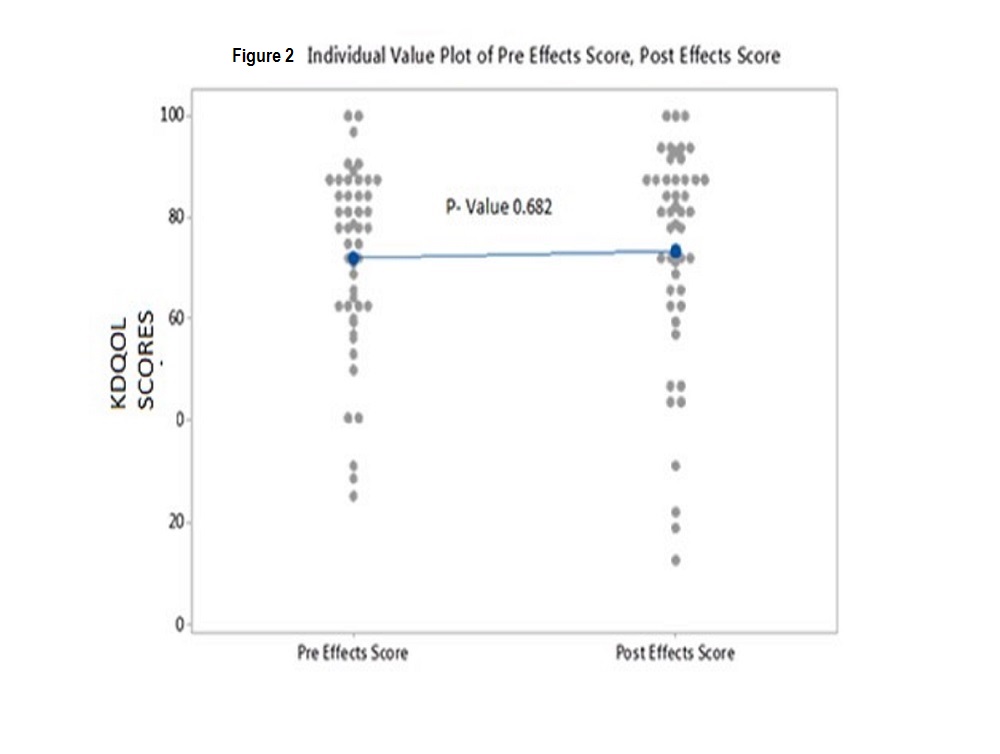
Figure2: Individual Value Plot of Pre Effects Score, Post Effects Score
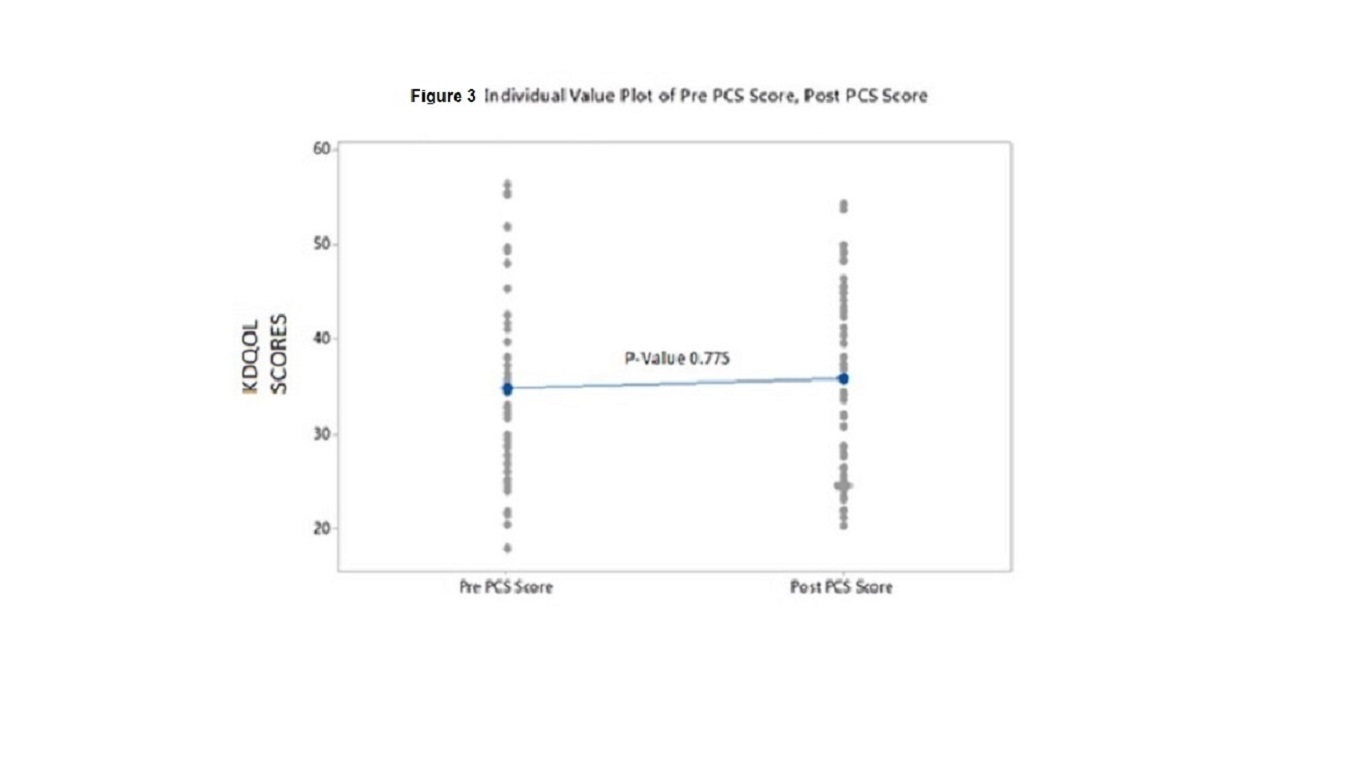
Figure3: Individual Value Plot of Pre Score, Post PCS Score
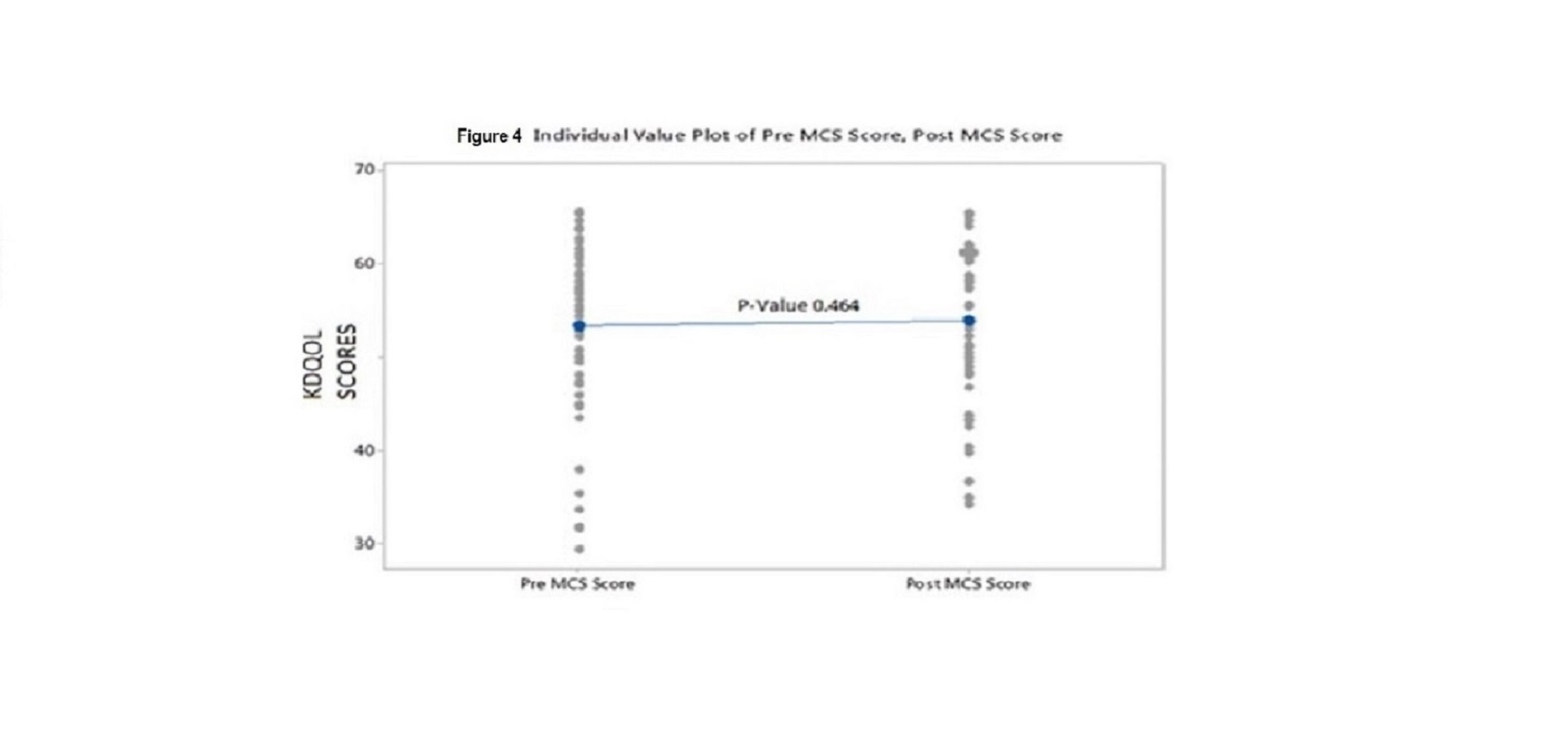
Figure4: Individual Value Plot of Pre MCS Score, Post MCS Score

Figure5: Individual Value Plot of Pre Burden Score, Post Burden Score
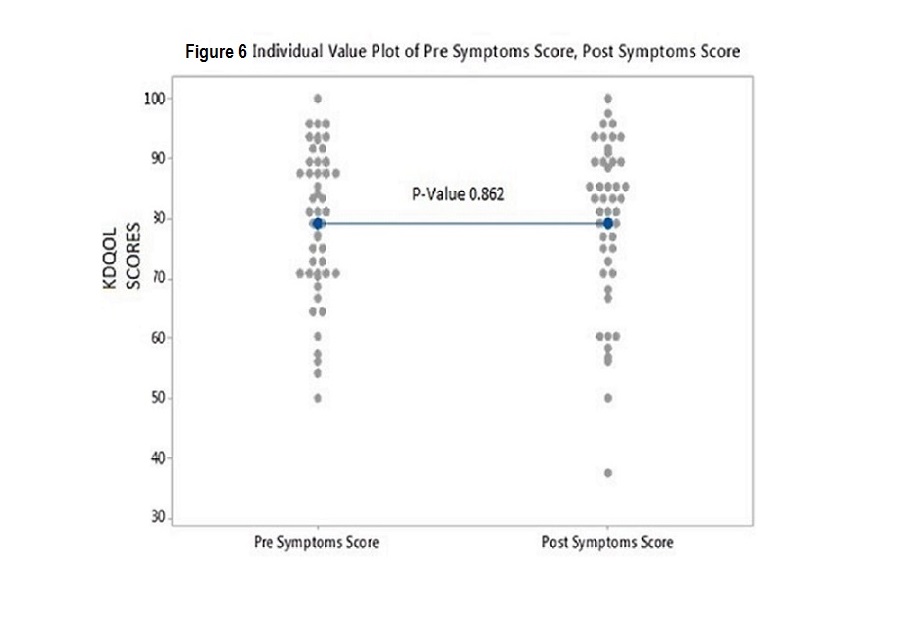
Figure6: Individual Value Plot of Pre Symptoms Score, Post Symptoms Score
Discussion
We examined the KDQOL-36 scores of 48 patients comparing their KDQOL scores prior to and after the implantation of our newly installed water treatment system capable of producing ultrapure water and dialysis fluid during the period between 08/10/2011-09/10/2014.We hypothesized that the use of UPDF will lead to the improved quality of life of patients on hemodialysis compared to patients dialyzed with conventional dialysate fluid.
We were unable to demonstrate a difference in KDQOL scores obtained before and after the implementation of our new ultrapure water system (see figures 2,3,4,5 and 6). The reason that we did not see an improvement in KDQOL scores after implementation of our new water system is unclear. Previous studies have demonstrated that quality of life scores may naturally decline over time in patients on dialysis for several years, or as patients age [13-15]. One possible reason that we were unable to detect a difference in scores, is that the KDQOL scores might have been naturally declining, from prior to the implementation of the UP water system to after wards as patient’s quality of life worsened over time. If this were the case then the fact that the KDQOL scores did not worsen might indicate an actual improvement compared to what would have been expected without the patients being dialyzed with our water system. Therefore, the use of UPDF may have positively impacted the KDQOL scores by slowing down the declination of scores. We did look back at prior data to examine this and did not find that each of the KDQOL scores was declining on the average for each patient over time (data not shown).
According to our study, it is unclear whether use of ultrapure dialysate will improve quality of life in dialysis patients. Improving or maintaining the quality of life of dialysis patients is one of the most important goals of dialysis therapy. This goal may be equally as important as improving survival and reducing hospitalizations of dialysis patients. The quality of life of dialysis patients is likely a more important goal than the improvement of process measures such as anemia management or AV fistula prevalence. Anything that can be done to improve patient’s quality of life or slowing worsening of quality of life on dialysis should be considered. Implementation of an ultrapure water treatment system is one intervention that we hoped would help to improve the quality of life of our dialysis patients. However, our study failed to confirm this benefit of ultrapure dialysate.
Conclusion
Our study failed to show an improvement in KDQOL scores in hemodialysis patients after implementation of water treatment system capable of producing ultrapure dialysate. However, given all the other potential benefits of ultrapure dialysis fluid as described above, including the reduction in infections and inflammation, use of ultrapure dialysis fluid compared to standard dialysate fluid still deserves further study, and should still be considered for use in dialysis facilities.
Acknowledgments:
Leslie Drager RN, BSN, CCRC
Jeff Quinto MBA, CPHQ, LSSBB
Ed Smith, Biomed Tech III
Kathy Duquette, LICSW
Gail Putin LICSW, MSW
References
- Ward RA (2007) World wide water standards for hemodialysis. Hemodial Int 11: S18.
- Ultrapure dialysate for hemodialysis and related therapies. AAMI Technical Information Report, 2011.
- Canaud B, Bosc JY, Leray H, Morena M, Stec F (2000) Microbiologic purity of dialysate: rationale and technical aspects. Blood Purif 18: 200-213.
- Ward RA (2000) Ultrapure dialysate: a desirable and achievable goal for routine hemodialysis. Semin Dial 13(6): 378-80.
- Center for Medicaid and State Operations/Survey & Certification Group
- Canaud B, Lertdumrongluk P (2012) Ultrapure Dialysis Fluid: A New Standard for Contemporary Hemodialysis. Nephrourol Mon 4: 519-523.
- Schiffl H, Wendinger H, Lang SM (2002) Ultrapure dialysis fluid and response to hepatitis B vaccine. Nephron 91: 530-531.
- Schiffl H, Lang SM, Fischer R (2002) Ultrapure dialysis fluid slows loss of residual renal function in new dialysis patients. Nephrol Dial Transplant 17: 1814-1818.
- Ouseph R, Jones S, Dhananjaya N, Ward RA (2007) Use of ultrafiltered dialysate is associated with improvements in haemodialysis-associated morbidity in patients treated with reused dialysers. Nephrol Dial Transplant 22: 2269-2275.
- Susantitaphong P, Riella C, Jaber BL (2013) Effect of ultrapure dialysate on markers of inflammation, oxidative stress, nutrition and anemia parameters: a meta-analysis. Nephrol Dial Transplant 28: 438-46. Your health and well-being kidney disease and quality of life (KDQOLTM-36).
- Chao S, Yen M, Lin TC, Sung JM, Wang MC, et al (2016) Psychometric properties of the Kidney Disease Quality of Life-36 Questionnaire (KDQOL-36™). West J Nurs Res 38: 1067-1082.
- Mujais SK, Story K, Brouillette J, Takano T, Soroka S, et al (2009) Health-related quality of life in CKD Patients: correlates and evolution over time. Clin J Am Soc Nephrol 4: 1293-1301.
- Bakewell AB, Higgins RM, Edmunds ME (2002) Quality of life in peritoneal dialysis patients: Decline over time and association with clinical outcomes. Kidney Int 61: 239-248.
- van Loon I, Hamaker ME, Boereboom FTJ, Grooteman MPC, Blankestijn PJ, et al (2017) A closer look at the trajectory of physical functioning in chronic hemodialysis. Age Ageing 6: 1-6.