Research Article
Sulafa Tageldin Abdel Rahman1, Abdalla Ahmed Elbashir2,*, Mohamed El-Mukhtar1 and Mohamed Mustafa Ibrahim3
1Chemistry Department, Faculty of Science, University of Sudan for Science and Technology, Sudan
2Chemistry Department, Faculty of Science, University of Khartoum, Sudan
3Chemistry Department, Faculty of Science and Arts in Mukwah, University of Al-Baha, Saudi Arabia
Corresponding author
*Corresponding author: Abdalla Ahmed Elbashir, Chemistry Department, Faculty of Science, University of Khartoum, Sudan, E-mail: aaelbashir@uofk.edu
Received Date: 11th June 2015
Accepted Date: 04th August 2015
Published Date: 07th August 2015
Citation
Abdel Rahman ST, Elbashir AA, El Mukhtar M, Ibrahim MM (2015) Development and Validation of Spectrophotometric Method for Determination of Thiamine (VB1) in Pharmaceutical Formulations using 1,2-Naphthoquine-4-Sulphonate (NQS). Enliven: Bio Anal Techniques 2(1): 001.
Copyright
@ 2015 Abdalla Ahmed Elbashir. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, that permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.in any medium, provided the original author and source are credited. reproduction in any medium, provided the original author and source are credited.
Abstract
Simple and sensitive spectrophotometric method for the determination of thiamine (VB1) in pharmaceutical formulations has been reported. The proposed method is based on the reaction between the (VB1) and sodium 1,2-naphthoquine-4-sulphonate (NQS) at alkaline medium (pH 11) to form deep brown product. Beer’s law is obeyed in the range 10-40 µg/ml of thiamine at maximum wavelength of 487 nm. Under optimized reaction conditions, linear regression equation of the calibration curve is: A= 0.022x + 0.171 (µg/ml) with a linear correlation coefficient of 0.997. The limit of detection (LOD) and limit of quantification (LOQ) were found to be 1.71 µg/mL and 5.18 µg/ml respectively. The method has been successfully applied to the determination of thiamine (VB1) in pharmaceutical formulations, and can be used as a replacement of the existing sophisticated method used in quality control laboratories.
Keywords
Thiamine (VB1); Spectrophotometric; Pharmaceutical formulation; Method validation; Sodium 1,2-naphthoquine-4-sulfonate (NQS)
Abstract
Simple and sensitive spectrophotometric method for the determination of thiamine (VB1) in pharmaceutical formulations has been reported. The proposed method is based on the reaction between the (VB1) and sodium 1,2-naphthoquine-4-sulphonate (NQS) at alkaline medium (pH 11) to form deep brown product. Beer’s law is obeyed in the range 10-40 μg/ml of thiamine at maximum wavelength of 487 nm. Under optimized reaction conditions, linear regression equation of the calibration curve is: A= 0.022x + 0.171 (μg/ml) with a linear correlation coefficient of 0.997.
The limit of detection (LOD) and limit of quantification (LOQ) were found to be 1.71 μg/mL and 5.18 μg/ml respectively. The method has been successfully applied to the determination of thiamine (VB1) in pharmaceutical formulations, and can be used as a replacement of the existing sophisticated method used in quality control laboratories.
Introduction
Thiamine hydrochloride (vitamin B1, a water-soluble vitamin), is a natural nutrient present in food and also added as an essential nutrient. It has been used for the prevention and treatment of beriberi, neuralgia, etc. in medical doses or as vitamin B1-enriched food or drinks [1]. It is necessary for carbohydrate metabolism and for the maintenance of neural activity [2]. Thiamine is one of the most important natural compounds of the B complex. It is named B1 because it was the first B vitamin discovered. All B vitamins help the body to convert food into glucose, which is “burned” to produce energy. These B vitamins help the body metabolize fats and protein. Thiamine is found in both plants and animals and plays a crucial role in certain metabolic reactions [3,4].
Since its discovery and isolation, there have been numerous reports on the determination of thiamine by capillary electrophoresis [5], chromatographic [6], spectrophotometric [1,7], spectrofluorimetric [8,9], chemiluminescence [10], potentiometric [11] and UV-irradiation methods [12]. These methods, however, have the disadvantages of high cost and require expensive sophisticated instrumentation, besides tedious experimental steps before determination.
Spectrophotometry is considered the most convenient analytical technique, because of its inherent simplicity, low cost, and wide availability in most quality control laboratories. Some spectrophotometric methods were reported for determination of thiamine in pharmaceutical formulations [1,7]. However these methods were associated with some major drawbacks such as pre-treatment oxidation by expensive chemicals, pre-concentration or a sequence of multiple reactions is required before color development.
NQS has been used as a color-developing reagent in the spectrophotometric determination of many pharmaceutical amines [13-27]. In depth review on the applications of NQS for determination of pharmaceutical bearing amine group have been reported by Elbashir et al. [24]. The reaction between NQS and thiamine has not been investigated yet. Therefore, the present study was devoted to investigate the reaction between NQS and thiamine, using this color reaction in the development of simple rapid spectrophotometric method for determination of thiamine in its dosage form.
Experimental
Apparatus
Absorbance was carried out by using UV-Visible spectrophotometer model Shimadzu mini 1240 with quartz cells of 1cm optical path length, pH meter was used for pH measurements, Analytical Balance and ultrasonic bath.
Material and Reagent
Thiamine hydrochloride was obtained from Autama Development company Ltd, Khartoum, Sudan (agent of Sigma-Aldrich), and used as received; its purity was 99.15%. A solution of sodium-1,2-naphthoquinone-4-sulfonate (NQS) 0.6% (w/v) was prepared by dissolving 0.6 g in distilled water, transferred into a 100 mL volumetric flask and diluted to the mark with distilled water and mixed well. The solution was freshly prepared and protected from light during use; buffer solution of pH 11.0 was prepared by mixing 100 ml of 0.1M aqueous solution of sodium bicarbonate with 50 ml of 0.1M solution of sodium carbonate and adjusted to pH 11.0 with 1M sodium hydroxide. All other chemicals were of analytical grade.
Preparation of Standard and Sample Solution
Stock standard solution of thiamine (1000 μg/mL)
An accurately 250 mg of thiamine hydrochloride standard was dissolved in distilled water, transferred into 250 ml volumetric flask, diluted to the mark with same solvent and mixed well. This stock solution was further diluted to obtain working solutions in the range of 10-40 μg/mL.
Sample solution
Five capsules (Thiamine 100 mg/capsule) were weighted and finely-grinded. A portion of the powder equivalent to 25 mg of the drug was weighed and dissolved in distilled water, filtered and then transferred into 250 volumetric flasks, completed to the mark with distilled water to give a solution of 100 µg/mL. The solution was then analyzed by the following procedure.
Procedure
A 1.0 mL of 100 μg/mL of thiamine was transferred into 10 ml volumetric flask, 2.0 mL of 0.6% (NQS) was added and followed by 2.0 mL pH 11.0 (Na2CO3/NaHCO3) buffer solution. The reaction was completed to volume with distilled water, and the resulting solution was measured at 487 nm against reagent blank treated similarly.
Job’s method
The Job’s method of continuous variation was employed to determine the stoichiometric ratio of the reaction. Master equimolar (5×10-3 M) aqueous solution of thiamine hydrochloride and NQS were prepared. Series of 10 mL portions of the master solution of thiamine and NQS were made up comprising different complementary proportions (1:9,…9:1, inclusive) in 10 mL volumetric flask containing 2 mL of buffer solution (pH=11.0). The solution was further manipulated as described under the general recommended procedures.
Results and Discussion
Absorption Spectra
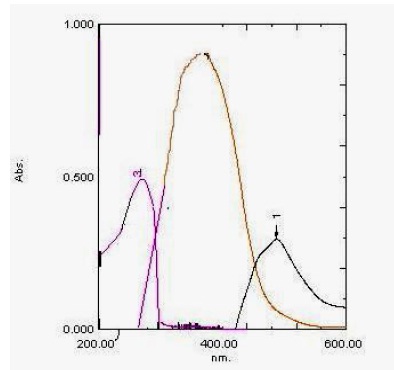
The absorption spectrum of thiamine was recorded against water (Figure 1), it was found that thiamine exhibits a maximum absorption peak (λ max) at 232 nm. Because of highly blue shifted λ max of thiamine, its determination in the dosage form based on the direct measurement of its absorption for ultraviolet is susceptible to potential interferences from the common excipients. Therefore, derivatization of thiamine red–shifted light-absorbing derivative was necessary. The reaction between thiamine and NQS was performed, and the absorption spectrum of the product was recorded against reagent blank (Figure 1). It was found that the product is brown colored exhibiting λmax at 487 nm, and the λmax of NQS was 361 nm. The λmax of thiamine-NQS derivative was red-shifted, eliminating any potential interference. Therefore, the measurements were carried out at 487 nm.
Figure 1. Absorption spectra of (1) finasteride (15 μg/ml) against a mixture of methanol and water blank, (2) NQS (0.006% (w/v) against water blank, (3) the reaction product of finasteride with NQS against reagent blank
Optimization of the Reaction Conditions
The optimum conditions for the development of method were established by varying the parameters one at a time while keeping the others fixed and observing the effect produced on the absorbance of the colored product. In order to establish experimental conditions, the effect of various parameters such as pH, time, buffer volume and concentration of NQS were investigated.
Effect of pH
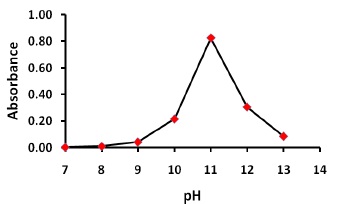
The effect of pH on the reaction between thiamine and NQS was examined by varying pH form 7.0 to 13.0. As shown in Figure 2, the absorbance of the product is low at pH 7.0, indicating that thiamine has difficulty to react with (NQS) in neutral media. This was possibly due to the existence of the amino group of thiamine in the form of hydrochloride salt, thus it loses its nucleophilic substitution capability. As the pH increased from 7 to 13, the readings increased rapidly, and the amino group of thiamine turns into the free amino group, thus facilitating the nucleophilic substitution. The maximum readings were attained at pH value of 11.0. At pH values more than 11.0 decrease in the readings occurred. This was attributed probably to the increase in the amount of hydroxide ion that holds back the reaction of thiamine with NQS. Reaction of NQS with compound bearing primary amines at pH 11.0 was reported [28].
Figure 2. Effect of pH on the reaction of thiamine with NQS, 1.0 mL of thiamine (100 μg/mL), 2.0 mL buffer solution (pH 11) , 2.0 mL NQS (0.6 w/v %), reaction time:15 min.
Effect of Time
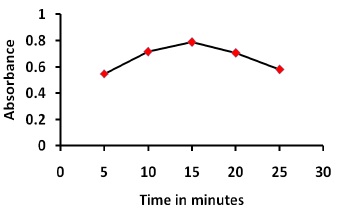
The absorbance of the reaction product was determined at different time (Figure 3). Keeping other conditions unchanged, the absorbance of the reaction product was measured after standing for different time periods at 25°C. The results show that thiamine react with NQS at 25°C and the absorbance begins to increase instantly and becomes constant after 15 min. Furthermore, it is also observed that the absorbance remain constant for 30 min.
Figure 3. Effect of standing time on the reaction of thiamine with NQS, 1.0 mL of thiamine (100 μg/mL), 2.0 mL buffer solution ( pH 11), 2.0 mL NQS (0.6 w/v %), reaction time:25 min.
Effect of Amount of the Buffer
Keeping pH at 11.0, the effect of amount of buffer solution on the absorbance of reaction product was also studied (data not shown). It shows that the absorbance of the reaction product enhances rapidly with the rise of amount of buffer solution, and becomes maximal when the amount of buffer solution is 2.0 mL. Therefore, the amount of 2.0 mL buffer solution was selected to ensure the highest absorbance.
Effect of NQS Concentration
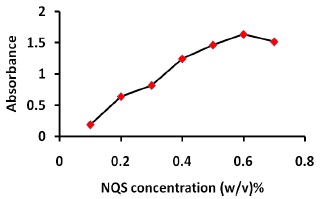
The studying of NQS concentrations revealed that the reaction was dependent on NQS reagent. The highest absorption intensity was attained at NQS concentration of 0.6% (w/v), and higher concentration of NQS (1.0%, w/v) had no effect on the absorption values, as shown in Figure 4.
From the above experiments, the optimized conditions used for the assay were: pH 11.0, NQS concentration 0.6% w/v, volume of the buffer 2.0 mL, reaction time 15 min and temperature 25ºC.
Figure 4. Effect of NQS concentration on the reaction of thiamine with NQS, 1.0 mL of thiamine (100 μg/mL), 2.0 mL buffer solution (pH 11), 2.0 mL NQS (0.6 w/v %), reaction time,15 min.
Validation of Method
The method was validated for the following parameters: specificity, linearity, precision, accuracy, limit of detection (LOD), limit of quantitation (LOQ), and robustness according to the International Conference on Harmonization (ICH) guidelines [28].
Linearity, Limit of Detection (LOD) Limit of Quantification (LOQ)
The linearity was evaluated by linear regression analysis determined by constructing seven concentrations of thiamine, in the range of 10–40 μg/mL, which was calculated by the least square regression method to calculate the calibration equation and the correlation coefficient. The calibration curves were constructed by plotting concentration versus absorbance, using linear regression analysis. The regression equation for the results was A=0.022x + 0.171 (r2=0.997), where A is the absorbance at 487 nm, x is the concentration of thiamine in μg/mL in the range of 10-40 μg/mL, and r is correlation coefficient (Table 1). The limit of detection (LOD) and limit of quantification (LOQ) were determined according to the following formula LOD=3.3×SDa/b, and LOQ=10×SDa/b, SDa is the standard deviation of the blank, b is the slope under the ICH guidelines. The LOD and LOQ were found to be 1.71 and 5.18 μg/mL, respectively (Table 1).
| Parameter | Value |
| Measurement wavelength (nm) | 487 |
| Linear range ( μg/mL) | 10-40 |
| Intercept | 0.171643 |
| Standard deviation of the Blank | 0.000455 |
| Slope | 0.022155 |
| Correlation coefficient (r) | 0.997 |
| Limit of detection, LOD ( μg/mL) | 1.71 |
| Limit of quant., LOQ ( μg/mL) | 5.18 |
Table 1. Parameters for the performance of the proposed method
Accuracy
The accuracy of the proposed method was carried out by applying 3 different concentrations 10, 20, and 30 μg/mL of thiamine drug within linear range calculated as the percentage of the drug recovered from the samples (Table 2).
(μg/mL)th
| thSample No.th | thRecovery ± SD*th |
|---|---|
| th1th | th99.0% ± 0.01th |
| th2th | th100.2% ± 0.01th |
| th3th | th100.0% ± 0.01th |
Robustness
Robustness was examined by evaluating the influence of small variation in the method variables on its analytical performance. In these experiments, one parameter was changed whereas the others were kept unchanged, and the recovery percentage was calculated each time. It was found that small variation in the method variables did not significantly affect the procedures; recovery values were recorded in Table 3. This indicated the reliability of the proposed method to routine application for the analysis of thiamine.
| Parameter | Recovery (% ± SD) |
| Recommended conditions | 95.33±0.006 |
| NQS concentration (%, w/v) 0.58 | 102.37±0.016 |
| NQS concentration (%, w/v) 0.62 | 97.82±0.013 |
| Buffer solution (pH) 10.8 | 96.01±0.029 |
| Buffer solution (pH) 11.2 | 93.63±0.044 |
| Reaction time (min) 13 | 98.63±0.009 |
| Reaction time (min) 17 | 96.56±0.055 |
Reaction Mechanism
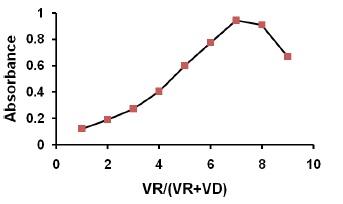
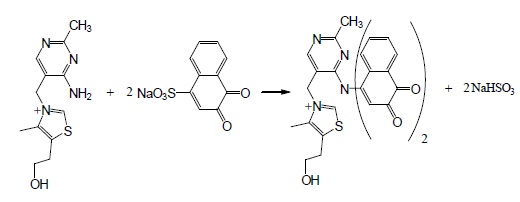
It has been reported that NQS could react with amino group of secondary amine derivative. Similarly, amino group of thiamine, taking on nucleophilicity due lone electron pair of nitrogen atom, trend to attack on the electron–deficient center in NQS, namely no.4 carbon atom (3,4-C=C carbon bond conjugate with 2-C=O, as a result 4-C of NQS becomes electron lacking center). At the same time, it has been proved that the composition of product is 1:2 of thiamine and NQS (Figure 5). So it is concluded that amino group of thiamine react with 4-sodium sulphonate of NQS molecule respectively, to form brown N-alkyl-amino-naphthoquinone. The reaction equation is shown in Figure 6.
Figure 5. The continuous variation plot for the stoichiometry of the reaction of thiamine with NQS
Figure 6. Scheme for the reaction pathway of thiamine with NQS
Application of the Proposed Method to Analysis of Thiamine Dosage Form
Thiamine tablets were subjected to the analysis by the proposed and the label claim agrees well with our new method as shown in Table 4. The proposed method has the advantage of being virtually free from interferences by excipients.
| Brand name of label claim (mg) | Amount found (mg) | (% found ± SD)a |
| Thiamine tablets (100 mg) | 95.55 | 95.33±0.006 |
Conclusion
The present paper described the evaluation of NQS as analytical reagents in the development of simple, sensitive, and accurate spectrophotometric methods, for the determination of thiamine in pharmaceutical formulation. The proposed method is simple, reliable, specific, accurate, reproducible, and highly sensitive, for the determination of thiamine in commercially available dosage forms. The procedure presented here does not need necessitate any expensive apparatus; therefore the proposed method can be used advantageously as a routine method for the determination of thiamine in quality control laboratories, our method can be applied to the determination of other secondary amine derivatives as well.
References
- Szpikowska-Sroka B (2013) A Simple and Sensitive Analytical Method for the Determination of Thiamine in Pharmaceutical Preparations. J Anal Chem 68: 218-222.
- Moszczyn Ski P, Pyc R (1998) Biochemistry of Vitamins. Warsaw: PWN.
- Roje S (2007) Vitamin B biosynthesis in plants, Phytochemistry 68: 1904-1921.
- Rocha FR, Fatibello Filho O, Reis BF (2003) A multicommuted flow system for sequential spectrophotometric determination of hydrosoluble vitamins in pharmaceutical preparations. Talanta 59: 191-200.
- Mrestani Y, Neubert RH (2000) Thiamine analysis in biological media by capillary zone electrophoresis with a high-sensitivity cell. J Chromatogr A 871: 351-356.
- Losa R, Sierra MI, Fernandez A, Blanco D, Buesa JM (2005) Determination of thiamine and its phosphorylated forms in human plasma, erythrocytes and urine by HPLC and fluorescence detection: a preliminary study on cancer patients. J Pharm Biomed Anal 37: 1025-1029.
- Ghasemi J, Abbasi B (2005) Simultaneous spectrophotometric determination of group B vitamins using parallel factor analysis: PARAFAC. J Chin Chem Soc 52: 1123-1129.
- Mohsen Z, Mohammad RG, Parviz N (2010) Dispersive liquid-liquid microextraction followed by spectrofluorimetry as a simple and accurate technique for determination of thiamine (vitamin B1). Microchim Acta 168: 317-324.
- Alonso A, Almendral MJ, Porras MJ, Curto Y (2006) Flow injection solvent extraction without phase separation. Fluorimetric determination of thiamine by the thiochrome method. J Pharm Biomed Anal 42: 171-177.
- Zhang C, Zhou G, Zhang Z, Aizawa M (1999) Highly sensitive electrochemical luminescence determination of thiamine. Anal Chim Acta 394: 165-170.
- Ciszewski A, Wang J (1992) Determination of thiamine by cathodic stripping voltammetry. Analyst 117: 985-988.
- Danet AF, Calatayud JM (1994) FIA-Spectrophotometric determination of thiamine after UV-irradiation. Talanta 41: 2147-2151.
- Hasani M, Yaghoubi L, Abdollahi H (2007) A kinetic spectrophotometric method for simultaneous determination of glycine and lysine by artificial neural networks. Anal Biochem 356: 74-81.
- Elbashir AA, Elwagee AHE (2012) Spectrophotometric determination of pyrimethamine (PYM) in pharmaceutical formulation using 1,2 naphthoquinone- 4-sulfonate (NQS). J Assoc Arab Univ Basic Appl Sci 11: 32-36.
- Elbashir AA, Abir AA, Shazalia MAA, Aboul-Enein HY (2012) 1,2-naphthoquinone-4-sulphonic acid sodium salt (NQS) as an analytical reagent for the determination of pharmaceutical amine by spectrophotometry. Spectr Rev 47: 219-232.
- Li QM, Yang ZJ (2007) Spectrophotometric determination of aminomethylbenzoic acid using sodium 1,2-naphthoquinone-4-sulfonate as the chemical derivative chromogenic reagent. Spectrochim Acta A Mol Biomol Spectrosc 60: 656-661.
- Xu L, Wang H, Xiao Y (2004) Spectrophotometric determination of ampicillin sodium in pharmaceutical products using sodium 1,2-naphthoquinone-4-sulfonic as the chromogentic reagent. Spectrochim Acta A Mol Biomol Spectrosc 60: 3007-3012.
- Ebraheem SAM, Elbashir AA, Abou-Enein HY (2011) Spectrophotometric methods for the determination of gemifloxacin in pharmaceutical formulations. Acta Pharma Sinica 4: 248-253.
- Ahmed SMA, Elbashir AA, Aboul-Enein HY (2015) New spectrophotometric method for determination of cephalosporins in pharmaceutical formulations. Arabian J Chem 8: 233-239.
- Elbashir AA, Ahmed SMA, Aboul-Enein HY (2012) New spectrofluorimetric method for determination of cephalosporins in pharmaceutical formulations. J Fluoresc 22: 857-864.
- Elbashir AA, Awad S (2013) A New Spectrophotometric Method for Determination of Penicillamine in Pharmaceutical Formulation Using 1, 2-naphthoquine-4-sulfonate (NQS). J Pharmacovigilance 1: 18-22.
- Elbashir AA, Ebraheem SAM, Elwagee AHE, Aboul-Enein HY (2013) New Spectrophotometric Methods for the Determination of Moxifloxacin in Pharmaceutical Formulations, Acta Chim Slov 60: 159-165.
- Altigani AMN, Elbashir AA (2014) Spectrophotometric Method for Determination of Primaquine in Pharmaceutical Formulations via Derivatization with 1, 2-Naphthoquinone-4-Sulfonate, Austin J Anal Pharm Chem 1: 1019.
- Abdalla FAA, Elbashir AA (2014) Development and Validation of Spectrophotometric Methods for the Determination of Mesalazine in Pharmaceutical Formulation. Med Chem 4: 361-366.
- Li Q, Zhang H (2008) A novel spectrophotometric method for the determination of aminophylline in pharmaceutical samples in the presence of methanol. Spectrochim Acta A Mol Biomol Spectrosc 70: 284-289.
- International Conference on Harmonization (ICH) (2005) Technical Requirements for the Registration of Pharmaceuticals for Human Use, Validation of analytical procedures: Text and methodology Q2.