Research Article
Peggy Matz1,2,3, Richard OC Oreffo4, and James Adjaye1,2*
1Institute for Stem Cell Research and Regenerative Medicine, Medical Faculty, Heinrich Heine University, 40225 Düsseldorf, Germany
2Max Planck Institute for Molecular Genetics, 14195 Berlin, Germany
3Institute of Biology, Humboldt University, 10099 Berlin, Germany
4Centre for Human Development, Stem Cells and Regeneration, Institute of Development Sciences, University of Southampton, Southampton SO16 6YD, United Kingdom
Corresponding author
James Adjaye, Chair for Stem Cell Research and Regenerative Medicine, Medical Faculty, Heinrich Heine University, 40225 Düsseldorf, Germany, Tel: (0211) 81- 08191; Fax: (0211) 81- 17858; E-mail: James.Adjaye@med.uni-duesseldorf.de
Received Date: 18th January 2016
Accepted Date: 11th March 2016
Published Date: 17th March 2016
Citation
Matz P, Oreffo RO, Adjaye J (2016) Culturing Human Pluripotent Stem Cells Using Human Fetal Femur Derived Mscs and Mouse Embryonic Fibroblasts: A Comparative Study. Enliven: J Stem Cells Regen Med 3(1):001.
Copyright
@ 2016 Dr. James Adjaye. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Numerous studies have shown the successful use of human stromal cells as feeders for the propagation of pluripotent stem cells. In this study, we
investigated the use of human fetal femur-derived mesenchymal stem cells (fMSCs) as an alternative to MEFs. We comparatively cultured over several
passages hESCs and iPSCs on fMSCs and MEFs as feeder layers as well as conditioned medium (CM) produced from both cell types. A quality check
for CM is the concentration of secreted Activin A, which is known to support self-renewal of pluripotent stem cells. The level of Activin A in fMSC-CM
was higher compared to MEF-CM when prepared under identical conditions. Furthermore, fMSCs secrete numerous factors amongst these are FGF2,
FGF19, VEGF, PDGF-AA, IL-11 which are involved in proliferation processes. The current study demonstrates that human fMSCs provide several
advantages over MEFs and can be used as an alternative for routine propagation of pluripotent stem cells. Use of fMSC obviates the unnecessary
need for breeding mice solely for the production of MEFs, therefore addressing the important issue of animal usage for MEF generation and most
importantly avoids the risk of contaminating human cells with mouse pathogens.
Introduction
In 1998 when Thomson and colleagues isolated and cultured the first human embryonic stem cell (hESC) line in vitro they used a mouse embryonic feeder (MEF) layer and fetal bovine serum (FBS)-containing culture medium [1]. Subsequently, culture conditions evolved as a consequence of the increased understanding of the molecular basis of pluripotency. FBS was replaced with knockout serum replacement (KSR) and FGF2 [2]. Subsequently, a MEF-conditioned medium was established for feeder-free cultivation of hESCs [3]. To avoid potential contamination with mouse cells and pathogens, human feeders have been used as a layer for culturing hESCs (fetal muscle and skin cells [4], adult skin cells [5], foreskin fibroblasts [6,7]). However, not all human fibroblast cell lines, indeed not all fibroblast lines of the same cell type support hESC growth [5]. In 2006, feeder-free and chemical defined culture conditions were published [8-12].
The derivation of induced pluripotent stem cells (iPSCs) from somatic cells comprises the use of a MEF layer [13,14]. MEFs support the maintenance of pluripotency by providing an extracellular matrix as well as expression of a range of adhesion molecules which support the attachment of pluripotent cells [15]. Additionally, MEFs express Activin A, Gremlin, TGFß1 and other factors which support self-renewal of hESCs [15-17]. Xu et al. published a protocol for producing MEF conditioned medium (MEF-CM) [3]. Supplementation of MEFs in culture with exogenous FGF2 stimulates these cells to secrete factors which support pluripotency and FGF2 supresses the expression and secretion of differentiation-inducing factors [3,16-18]. To date, the routine culture of ESCs as well as iPSCs still employs MEFs as feeder layers and/or conditioned medium (CM). We previously published an optimized protocol which describes the isolation and cultivation of MEFs as well as the preparation of MEF-CM [17].
Here, we present the use of fetal femur-derived mesenchymal stem cells (fMSCs) as a feeder layer and also conditioned medium derived from fMSCs-(fMSC-CM) in comparison to MEFs and MEF-CM. In contrast to MEFs or other human fibroblasts such ashuman foreskin fibroblast cells (HFF1), fMSCs display enhanced sustained proliferation and have significantly lower doubling times in comparison with adult-derived cultures. Additionally, fMSCs are fully characterized as mesenchymal stem cells and are already advocated for application in clinical approaches [19]. The human origin of fMSCs negates any concerns over potential contamination with mouse cells and pathogens associated with MEFs during cell culture and are attractive in terms of animal replacement, refinement and reduction strategies.
Material and Methods
Cell Lines and Culture
The human embryonic stem cell (hESCs) line H1 was bought from WiCell Research Institute. The iPSC lines were derived in our laboratory with an episomal approach [20,21]. The hESC and induced pluripotent stem cell (iPSC) lines were cultured on Matrigel-coated plates with MEFs serving as feeder layer with a medium consisting of Knock Out DMEM (Gibco/Life Technologies) supplemented with 20 % Knock Out Serum Replacement (Gibco/Life Technologies), 0.1 mM non-essential amino acids (Invitrogen/Life Technologies), 0.1 mM L-glutamine (Invitrogen/Life Technologies), 0.1 mM ß-Mercaptoethanol (Sigma-Aldrich), 0.5 % penicillin and streptomycin and 8ng/ml basic fibroblast growth factor (bFGF, Invitrogen/Life Technologies) as described [22].
Human fetal femur-derived mesenchymal stem cells (fMSCs; H1536) were obtained after day 55-post conception according to guidelines issued by the Polkinghome Report and with ethical approval from the Southampton &South West Hampshire Local Research Ethics Committee.
Mouse embryonic fibroblasts (MEFs) were isolated from a pregnant female mouse (CF-1, Harlan, USA). The mouse was sacrificed at 13 or 14 days post-cointum (d.p.c.) through cervical dislocation [17]. MEFs and fMSCs were cultured in Dulbecco`s modified Eagle medium (DMEM, Gibco/Life Technologies) containing 10 % fetal bovine serum (FBS, Invitrogen/Life Technologies) and 0.5 % penicillin and streptomycin (Invitrogen/Life Technologies) [22].
Production of Conditioned Medium
Both fMSCs and MEFs were inactivated with mitomycin c (Sigma-Aldrich) and plated in a density of 56,000 cells/cm2. One day after plating the medium was exchanged with hESC medium (unconditioned medium, UM) and supplemented with 4 ng/ml of FGF2. Conditioned medium was collected 24 hours later and additional fresh UM containing 4ng/ml of FGF2 was added to the cells. This procedure was repeated for the next six days. The CM collected from all the days were combined and sterilized by filtration. 50 ml aliquots were stored at -80°C. Prior to using the CM, the stocks were thawed and supplemented with 4ng/ml of FGF2 (Peprotech) before adding to cells growing on Matrigel [17].
Immunofluorescence-Based Detection of Expressed Proteins
Cells were fixed with 4 % paraformaldehyde for 20 min and washed twice with PBS. Permeabilisation was achieved by incubating the cells with 0.1 % Triton X-100 in PBS for 10 min. Afterwards, the cells were blocked with a solution consisting of 10 % fetal bovine serum (Invitrogen), 0.1 % Triton X-100 (Sigma) in PBS for 45 min. Next, the cells were washed with PBS twice for 5 min per wash. The primary antibody was diluted in the blocking solution. The cells were incubated with primary antibody solution overnight at 4°C. Next day the cells were washed three times with PBS for 5 min per wash. The secondary antibody was diluted in blocking solution. The cells were incubated with the secondary antibody for 1 hour at RT in the dark. All antibodies are listed in Table 1. Subsequently, the cells were washed three times with PBS for 5 min per wash. The nuclei of the cells were counter-stained with 4´,6-Diamidin-2-phenylindol (DAPI, Invitrogen) solution (200 ng/ml in PBS) in the dark for 20 min at RT. Finally, the cells were covered with PBS to keep them moist. The fluorophores on the secondary antibodies were visualised using a Zeiss LSM 510 Meta confocal microscope with a connected camera for microscopy model AxioCam ICC3 and the software Axiovision 4.6.
|
Primary antibodies |
||||
|
Human antigene |
Species raised in |
Company |
Catalog Nr. |
Dilution |
|
OCT4 |
mouse |
Santa Cruz |
sc-5279 |
1:200 |
|
SOX2 |
goat |
Santa Cruz |
sc-17320 |
1:200 |
|
NANOG |
goat |
R&D Systems |
AF1997 |
1:200 |
|
TRA-1-60 |
mouse |
Millipore |
SCR001 |
1:200 |
|
Brachyury (T) |
goat |
R&D Systems |
AF2085 |
1:200 |
|
SOX17 |
goat |
R&D Systems |
AF1924 |
1:100 |
|
Nestin (NES) |
mouse |
Millipore |
MAB5326 |
1:200 |
|
Secondary antibodies |
||||
|
Antigene |
Species raised in |
Company |
Catalog Nr. |
Dilution |
|
anti-goat IgG, |
donkey |
Invitrogen |
A11055 |
1:300 |
|
anti-goat IgG, |
chicken |
Invitrogen |
A21468 |
1:300 |
|
anti-mouse IgG, |
goat |
Invitrogen |
A11001 |
1:300 |
|
anti-mouse IgG, |
goat |
Invitrogen |
A11005 |
1:300 |
Table 1: List of Antibodies used for Immunofluorescence-Based Detection of Protein Expression
RNA Isolation and Quantitative Real-Time PCR
RNA isolation was carried out using the Direct-zol RNA Miniprep (Zymo Research) or Universal RNA Purification Kit (Roboklon) adhering tothe manufacturer’s guidelines. Reverse transcription was done by using moloney murine leukemia reverse transcriptase (Promega). Comparative quantitative real-time PCR (qRT-PCR) was performed in 384-well Optical Reaction Plates (Applied Biosystems) using the SYBR® Green PCR Master Mix (Applied Biosystems).The primer sequences are listed in Table 2. Expression levels of pluripotent-associated marker genes were normalized to GAPDH expression and calculated by the ΔΔCt method. Statistical differences were compared by unpaired Student’s ?-tests using the In Statistical application (GraphPad Software). Significance was defined as ?< 0.05, with error bars corresponding to standard error of the mean.
|
Gene |
Size (bp) |
Primer |
Sequence 5`-3` |
|
OCT4 |
119 |
forward |
GTGGAGGAAGCTGACAACAA |
|
|
|
reverse |
ATTCTCCAGGTTGCCTCTCA |
|
GAPDH |
81 |
forward |
CTGGTAAAGTGGATATTGTTGCCAT |
|
|
|
reverse |
TGGAATCATATTGGAACATGTAAACC |
|
SOX2 |
78 |
forward |
GTATCAGGAGTTGTCAAGGCAGAG |
|
|
|
reverse |
TCCTAGTCTTAAAGAGGCAGCAAAC |
|
NANOG |
78 |
forward |
CCTGTGATTTGTGGGCCTG |
|
|
|
reverse |
GACAGTCTCCGTGTGAGGCAT |
|
LEFTY1 |
76 |
forward |
AATGTGTCATTGTTTACTTGTCCTGTC |
|
|
|
reverse |
CAGGTCTTAGGTCCAGAGTGGTG |
|
GDF3 |
96 |
forward |
TTGGCACAAGTGGATCATTGC |
|
|
|
reverse |
TTGGCACAAGTGGATCATTGC |
|
FGF4 |
109 |
forward |
CCCTTCTTCACCGATGAGTGC |
|
|
|
reverse |
CATTCTTGCTCAGGGCGATG |
|
DPPA4 |
91 |
forward |
TGGTGTCAGGTGGTGTGTGG |
|
|
|
reverse |
CCAGGCTTGACCAGCATGAA |
|
DNMT3B |
93 |
forward |
GCTCACAGGGCCCGATACTT |
|
|
reverse |
GCAGTCCTGCAGCTCGAGTTTA |
Table 2: List of Primer Sequences used for Quantitative Real-Time PCR.
Embryoid Body Formation
hESCs or iPSCs were seeded on ultra-low attachment dishes (Corning) and cultured in DMEM (Gibco/Life Technologies). The cells formed embryoid bodies (EBs). These EBs were cultured for seven days and then seeded onto 12 well cell culture dishes previously coated with 0.1 % gelatin. The EBs attached onto the gelatin surface and differentiated. Three days after seeding on gelatin some wells were fixed with paraformaldehyde to detect Brachyury (mesoderm). In order to detect specific proteins of the two germ layers endoderm and ectoderm the EBs were fixed with paraformaldehyde 10 days after seeding on gelatin. Generally, the EBs were stained by immunofluorescence-based staining of marker proteins of the three germ layers.
Quantification of Activin A Levels
When producing conditioned medium (CM) daily aliquots were taken for Activin A measurement. To measure levels of Activin A within the media the enzyme-linked immunosorbent assay (ELISA) -based Human/Mouse/Rat Activin A Immunoassay (R&D Systems) was used, as described [17].
Results
Comparative Culture with Feeders Derived from MEFs and fMSCs
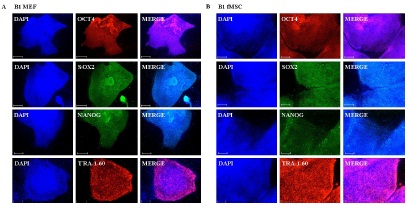
It is known that mouse embryonic fibroblasts (MEFs) support the maintenance of pluripotency by producing extracellular matrix as well as through the expression of a range of adhesion molecules and cytokines such as Activin A, Gremlin and TGFß1 which provide undifferentiated growth of hESCs [15-17]. Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) were comparatively cultured on MEF-feeder layer and fMSC-feeder layer over several passages. Immunofluorescence-based detection of expression of the pluripotency-associated proteins OCT4, SOX2, NANOG and TRA-1-60 showed expression under both conditions (Figure 1).
Figure 1: Immunofluorescence-based detection of human pluripotent specific proteins OCT4, SOX2, NANOG and TRA-1-60. The hESC line H1 as well as the iPSC lines were cultured on MEF layer (left panel) and on fMSC layer (right panel). This representative figure shows the staining of the iPSC line iHFF1-B1.
Expansion of Pluripotent Cells using Conditioned Medium
Feeder-free culture avoids potential contamination with mouse cells and pathogens. Feeders can be used to produce conditioned medium (CM) which contains the requisite factors which were secreted by the feeder layer. Following addition of exogenous FGF2, MEFs are stimulated to secrete factors which support pluripotency, e.g. Activin A, Gremlin and TGFß1 [3,15-18] and as a quality check of the produced CM an Activin A assay is routinely carried out [17].
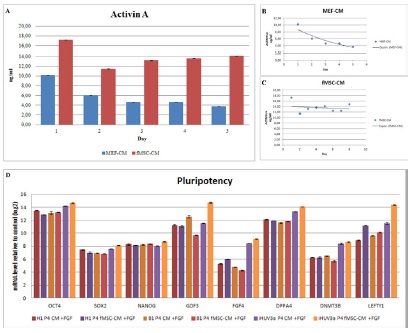
CM was prepared from MEF- and fMSC-feeders. Daily aliquots were taken to measure the level of Activin A in the conditioned medium. In general, fMSC-feeders secreted higher concentrations of Activin A than MEF-feeders (Figure 2). On day one the level of Activin A was observed to be 1.69-fold higher in fMSC-feeders compared to MEF-feeders. fMSC-feeders showed stable secretion of Activin A during a period of five days. In contrast, MEF-feeders secreted less Activin A and, furthermore, the level of Activin A was observed to continuously decline over the 5 day period (Figure 2 A-C).
In order to detect whether or not other cytokines were secreted by fMSCs a cytokine array was used to detect the factors secreted (data not shown). Amongst others fMSCs secrete FGF2, FGF19, VEGF, PDGF-AA and IL-11, which are involved in proliferation processes [16,23-26].
To analyse the expressed RNA level of pluripotency-associated genes, quantitative real-time PCR (qRT-PCR) was performed (Figure 2D). Comparable levels of gene expression could be detected in all samples (MEF-CM, fMSC-CM), with and without freshly supplemented FGF2.
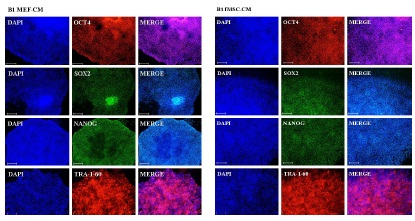
hESCs and iPSCs were compared following culture on Matrigel-coated plates with fMSC-CM or MEF-CM over several passages. Immunofluorescence-based staining of the pluripotency-associated proteins OCT4, SOX2, NANOG and the surface marker TRA-1-60 showed expression under both conditions (Figure 3).
Figure 2: Comparative quantification of Activin A levels secreted by MEFs and fMSCs. A) Comparison of Activin A secretion level of MEFs and fMSCs over 5 days. B) Activin A secretion level trend of MEFs over 5 days. C) Activin A secretion level trend of fMSCs over 8 days. D) Quantitative real-time PCR analysis of pluripotent-specific genes. The hESC line H1 as well as two iPSC lines (iHFF1-B1 and iHUVEC-3a) were cultured feeder-free with MEF conditioned medium (MEF-CM) plus additional FGF2 (MEF-CM +FGF). Comparative H1, iHFF1-B1 and iHUVEC-3a were cultured feeder-free with fMSC conditioned medium plus additional FGF (fMSC-CM +FGF). The expression of OCT4, SOX2, NANOG, GDF3, FGF4, DPPA4, DNMT3B and LEFTY was measured.
Figure 3: Immunofluorescence-based detection of expressed proteins. The hESC line H1 as well as the iPSC lines were cultured feeder-free with MEF conditioned medium (left panel) and with fMSC conditioned medium (right panel).This representative figure shows the staining of the iPSC line iHFF1-B1.
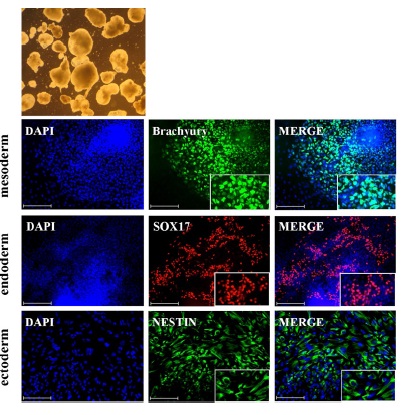
All cells, regardless of the culture condition employed, were able to differentiate into all three germ layers mesoderm, endoderm and ectoderm (Figure 4).
Figure 4: Spontaneous differentiation into germ-layer specific cell types by means of EB formation. In fMSC conditioned medium cultured pluripotent stem cells (hESC or iPSC) were used to form embryoid bodies (EB) and further differentiate the EBs into cell types of the three germ-layers (mesoderm, endoderm and ectoderm).This representative figure shows the staining of the iPSC line iHFF1-B1.
Discussion
The current study demonstrates the feasibility of using as an alternative to MEFs, fMSCs as a feeder layer or for producing conditioned medium (fMSC-CM) for expansion and maintenance of pluripotent cells (Figure 1 and Figure 3). All cell lines (hESC and iPSC) cultured on fMSC or in fMSC-CM maintained self-renewal and pluripotency. Additionally, hESCs and iPSCs following culture on fMSC or fMSC-CM were all able to differentiate into cell types representative of all three germ layers (Figure 4).
Secretion of Activin A from fMSCs on day one was 70 percent higher compared to levels observed for MEFs. In addition, secretion of Activin A by fMSCs was stably maintained over 5 days in contrast to MEFs (Figure 1A). Furthermore, the secretion of Activin A by fMSCs remained stable beyond five days. Examination of secreted Activin A by fMSCs over 8 days revealed constant levels which were significantly higher than levels secreted by MEFs (Figure 1B-C). These results indicate that fMSCs are much more potent for the production of conditioned medium over a longer culture period.
Measurement of secreted factors using a cytokine array revealed that fMSCs secrete FGF2, FGF19, VEGF, PDGF-AA and IL-11, which are involved in proliferation processes [16,23-27]. Fibroblast growth factor 2 (FGF2) modulates the activity of TGFβ superfamily members and is one of the most critical factors for maintaining pluripotency [16]. We previously demonstrated that IL-11 can maintain self-renewal and pluripotency in hESCs. Qualitative modeling identified IL-11 as a novel regulator for maintaining self-renewal in human pluripotent stem cells [27]. Furthermore, fMSCs secrete platelet-derived growth factor-AA (PDGF-AA) which together with FGF2 confers a synergistic effect on cell proliferation [25].
The proliferation rate of fMSCs is enhanced and, in addition, fMSCs display significantly lower doubling times in comparison with adult-derived cells [19]. In contrast to MEFs or other human fibroblasts such asHFF1 cells, fMSCs can be expanded for longer periods.
In conclusion, we show for the first time that human fetal femur derived MSCs (fMSCs) are superior to mouse embryonic fibroblasts (MEFs) for the production of feeder cells and conditioned medium due to their enhanced secretion of Activin A, and other cytokines (FGF2, FGF19, VEGF, PDGF-AA and IL-11) known to support the proliferation of cells. These attributes endow fMSCs as a useful alternative to MEFs for routine and long-term propagation of pluripotent cells while addressing issues of mouse cell/pathogen contamination and animal replacement, refinement and reduction and offer a facile cell culture approach for the stem cell community.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgements
J.A. acknowledges support from the medical faculty of Heinrich-Heine University, Düsseldorf and the European Union funding/FP7,Grant Agreement n° 305299 (Aged Brain SYSBIO).R.O. is supported by grants from the BBSRC (BB/L021072/1 and BB/L00609X/1) and MRC (G1000842 and MR/L012626/1).
References
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282: 1145-1147.
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, et al. (2000) Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol 227: 271-278.
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, et al. (2001) Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotech 19: 971-974.
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A (2002) Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotech 20: 933-936.
- Richards M, Tan S, Fong CY, Biswas A, Chan WK, et al. (2003) Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells 21: 546-556.
- Amit M, Margulets V, Segev H, Shariki K, Laevsky I, et al. (2003) Human feeder layers for human embryonic stem cells. Biol Reprod 68: 2150-2156.
- Inzunza J, Gertow K, Stromberg MA, Matilainen E, Blennow E, et al. (2005) Derivation of human embryonic stem cell lines in serum replacement medium using postnatal human fibroblasts as feeder cells. Stem Cells 23: 544-549.
- Shin S, Mitalipova M, Noggle S, Tibbitts D, Venable A, et al. (2006) Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells 24: 125-138.
- Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, et al. (2006) Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 24: 185-187.
- Yao S, Chen S, Clark J, Hao E, Beattie GM, et al. (2006) Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A 103: 6907-6912.
- Amit M, Itskovitz-Eldor J (2006) Sources, derivation, and culture of human embryonic stem cells, Semin Reprod Med 24: 298-303.
- Skottman H, Hovatta O (2006) Culture conditions for human embryonic stem cells. Reproduction 132: 691-698.
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663-676.
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917-1920.
- Eiselleova L, Peterkova I, Neradil J, Slaninova I, Hampl A, et al. (2008) Comparative study of mouse and human feeder cells for human embryonic stem cells. Int J Dev Biol 52: 353-363.
- Greber B, Lehrach H, Adjaye J (2007) Fibroblast growth factor 2 modulates transforming growth factor beta signaling in mouse embryonic fibroblasts and human ESCs (hESCs) to support hESC self-renewal. Stem Cells 25: 455-464.
- Jozefczuk J, Drews K, Adjaye J (2012) Preparation of mouse embryonic fibroblast cells suitable for culturing human embryonic and induced pluripotent stem cells. J Vis Exp 64.
- Vallier L, Alexander M, Pedersen RA (2005) Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 118: 4495-4509.
- Mirmalek-Sani SH, Tare RS, Morgan SM, Roach HI, Wilson DI, et al. (2006) Characterization and multipotentiality of human fetal femur-derived cells: implications for skeletal tissue regeneration. Stem Cells 24: 1042-1053.
- Matz P, Adjaye J (2015) Generation of iPSC line epiHUVEC from human umbilical vein endothelial cells. Stem Cell Res 15: 581-583.
- Matz P, Adjaye J (2016) Episomal-based generation of iPSC line from human fetal foreskin fibroblasts. Stem Cell Res 16: 67-69.
- Wolfrum K, Wang Y, Prigione A, Sperling K, Lehrach H, et al. (2010) The LARGE principle of cellular reprogramming: lost, acquired and retained gene expression in foreskin and amniotic fluid-derived human iPS cells. PloS one 5: e13703.
- Wu X, Ge H, Lemon B, Vonderfecht S, Baribault H, et al. (2010) Separating mitogenic and metabolic activities of fibroblast growth factor 19 (FGF19). Proc Natl Acad Sci U S A 107: 14158-14163.
- Cheng X, Ying L, Lu L, Galvao AM, Mills JA, et al. (2012) Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell 10: 371-384.
- Kiso M, Hamazaki TS, Itoh M, Kikuchi S, Nakagawa H, et al. (2015) Synergistic effect of PDGF and FGF2 for cell proliferation and hair inductive activity in murine vibrissal dermal papilla in vitro. J Dermatol Sci 79: 110-118.
- Nguyen PM, Putoczki TL, Ernst M (2015) STAT3-Activating Cytokines: A Therapeutic Opportunity for Inflammatory Bowel Disease? J Interferon Cytokine Res 35: 340-350.
- Peterson H, Abu Dawud R, Garg A, Wang Y, Vilo J. et al. (2013) Qualitative modeling identifies IL-11 as a novel regulator in maintaining self-renewal in human pluripotent stem cells. Front Physiol 4: 303.