Research Poster
A Goltsov1*, SG Sokolovski2, and EU Rafailov2
1School of Science, Engineering and Technology, Abertay University, Dundee, United Kingdom
2Optoelectronics and Biomedical Photonics Group, AIPT, School of Engineering and Applied Sciences, Aston University, Birmingham, United Kingdom
Corresponding author
Alexey Goltsov, School of Science, Engineering and Technology, Abertay University, Dundee, United Kingdom, Tel: 44 (0)1382 308432; E-mail: A.Goltsov@abertay.ac.uk
Received Date: 15th July 2015
Accepted Date: 21st July 2015
Published Date: 24th July 2015
Citation
Goltsov A, Sokolovski SG, Rafailov EU (2014) OCoupled ROS and Ca2+ Sustained Activation in Cancer Cells Induced by Near-Infrared Laser Pulse: Application for Cancer Therapy. Enliven: Bioinform 2(1): 001.
Copyright
@ 2015 Dr. Alexey Goltsov. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Infrared quantum-dot laser diode irradiation (1268 nm) was observed to induce irreversible oxidative stress in cancer cells through direct triplet→singlet oxygen transition designating a novel cancer treatment equally with photodynamic therapy, PDT (Sokolovski, et al. Sci. Rep. 3:3484, 2013). A single laser pulse induction of reactive oxygen species (ROS) was attended with Ca2+ release and coupled ROS and Ca2+ sustained activation occurs far beyond the initial laser pulse exposure. Cancerous cells (HeLa) were observed to be more sensitive to laser-induced ROS generation then normal keratinocytes. The developed model of the laser-induced oxidative stress showed that the main impact on the cell oxidative state makes a cascade of secondary ROS triggered by primary laser-induced singlet oxygen and irreversible depletion of cellular antioxidant system in cancerous cells. We proposed the model of the amplification of laser-induced ROS generation due to the coupled ROS and Ca2+ activation through endoplasmic reticulum (ER) and mitochondria crosstalk.
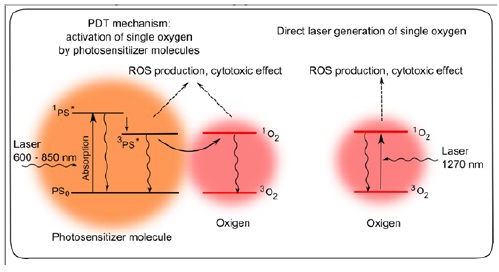
Direct Laser-Induced Oxidative Stress vs. Photodynamic Therapy
(Figure 1)
Kinetic model of redox homeostasis and its imbalance by laser-induced ROS generation in normal and cancerous cells
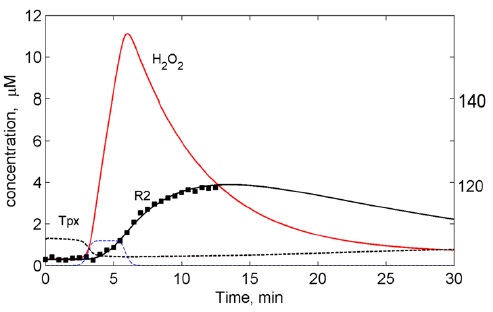
The model takes into account the following processes: (i) endogenous generation of primary ROS (O2?, 1O2) and laser-produced 1O2,; (ii) their transformation into H2O2 by superoxide dismutase (SOD); (iii) generation of a secondary pool of ROS from H2O2 through the Fenton reactions (R2); and (iv) scavenging of H2O2 and its by-products by the cellular antioxidant system. In the enzymatic submodel of the H2O2 degradation is based on the redox cascade reactions which correspond to the key antioxidant cellular systems: thioredoxin peroxidase/thioredoxin/thioredoxin reductase (Tpx/Trx/TR) and glutathione peroxidase/glutathione/glutathione reductase (Gpx/GSS/GR) systems (Sokolovski, et al. Sci. Rep. 3: 3484, 2013) (Figures 2-10).
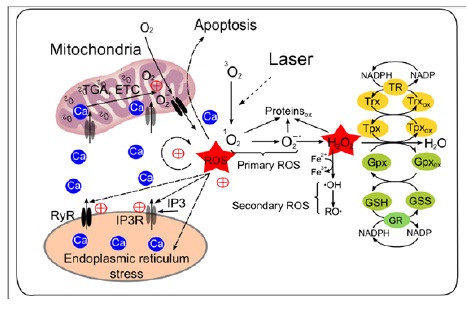
Figure 2: Model of laser- induced oxidative stress and mechanism of ROS amplification due to the coupled ROS and Ca2+ activation
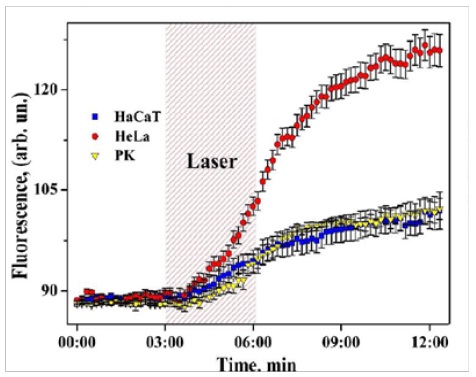
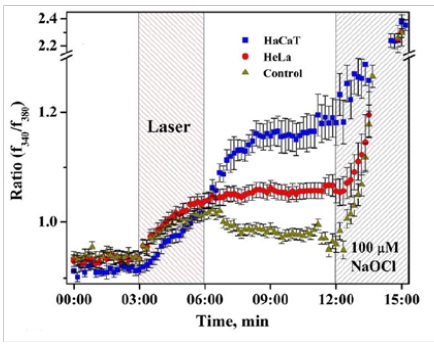
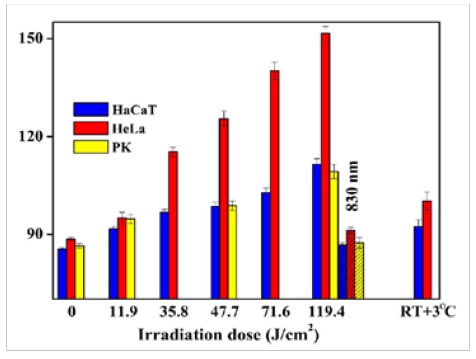
Figure 3,4: Coupled ROS (left) and Ca2+ (right) sustained activation induced by low energy near-infrared continuous wave laser pulse (1265 nm, 3 min, <200 J/cm2) in HaCaT (epidermal keratinocytes), HeLa (cervical cancer cells), and PK (primary keratinocytes) (Sokolovski, et al. Sci. Rep. 3 : 3484, 2013).
Figure 5: Dose-dependency of ROS concentration (fluorescence) in cell lines (12th min). 830 nm laser irradiation taken as a negative control.
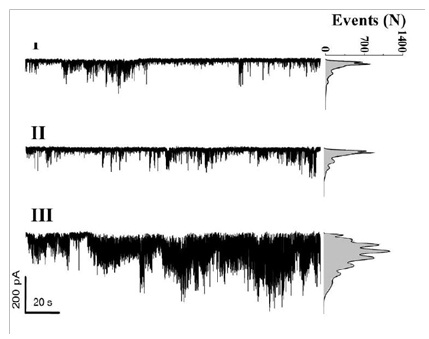
Figure 6: Single Ca2+ channel currents recorded before (I), during (II), and after (III) 1268 nm laser irradiation of 47.7 J/cm2. Right: opened channel events amplitude.
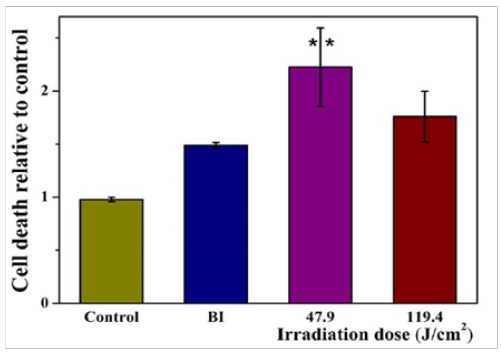
Figure 7: Laser-triggered cancer cell death. HeLa cell death rate measured by an enzymatic assay of LDH release.
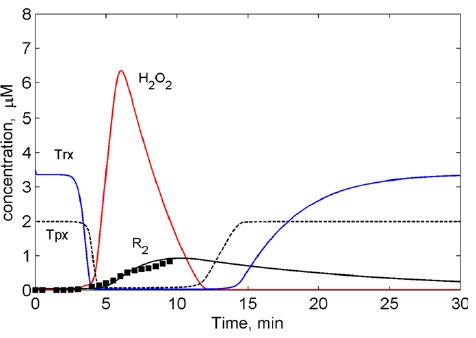
Figure 8: Results of the computational modelling: Suppression of laser-induced oxygen radicals (RO?) in normal cells by antioxidant cellular system (Tpx/Trx). Points ? experimental data.
Figure 9: Results of the computational modelling: Sustained oxygen radical (RO?) induced by laser. Depletion of the antioxidant cellular system (Tpx/Trx) by laser-induced ROS. Points ? experimental data.
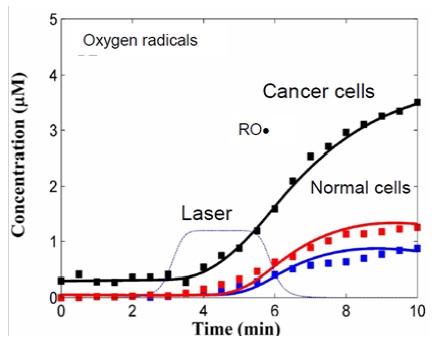
Figure 10: Sensitivity of cancer cells to laser-induced oxidative stress. Computational modelling of ROS generation by laser-induced single oxygen in normal cells (red, blue) and cancerous cell (black) at the different rate of single oxygen generation V1 (blue, black) and V2>V1 (red). Points ? experimental data.
Conclusions
The joint in vitro and in silico investigation revealed hypersensitivity of cancer cells to 1268 nm laser-induced oxidative stress. The proposed amplification mechanism of laser-induced ROS due to the coupled ROS and Ca2+ activation through ER and mitochondria crosstalk is suggested to cause apoptosis in cancerous cells not damaging normal cells. The obtain results may propose a novel therapeutic approach based on direct laser photoactivation of molecular oxygen in the tumour without the need for exogenous drugs and gain opportunity to develop PS-free cancer phototherapy.
Acknowledgments
FP7 FAST-DOT and SICSA (Scottish Informatics and Computer Science Alliance).