Research Article
Lucinda M Lamb1, Jennifer Tabor-Rennie1, Jared L Crandon1, and David P Nicolau1,2*
1Center for Anti-Infective Research and Development
2Division of Infectious Diseases, Hartford Hospital, Hartford, CT
Corresponding author
David P. Nicolau, PharmD, FCCP, FIDSA, Center for Anti-Infective Research and Development, Hartford Hospital 80 Seymour Street, Hartford, CT 06102, Tel: 860-972-3941; Fax: 860-545-3992; E-mail: david.nicolau@hhchealth.org
Received Date: 08thAugust 2015
Accepted Date: 07th September 2015
Published Date: 11th September 2015
Citation
Lamb LM, Tabor-Rennie J, Crandon JL, Nicolau DP (2015) Comparison of Mechanical Tissue Grinders and the Mini Beadbeater 96 for Homogenization of Murine Lung and Thigh Tissue for Subsequent Quantitation of Bacterial Density. Enliven: Microb Microbial Tech 1(2): 001.
Copyright
@ 2015 Dr. David P. Nicolau. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Quantitation of bacterial density in animal tissues is commonplace in pre-clinical antibacterial drug discovery and development research. Routinely used
tissue homogenization methods are labor intensive, time consuming processes that can lead to repetitive motion related injuries to laboratory personnel.
Findings:
In this study, we compared our standard method of tissue homogenization, mechanical tissue grinders, to the Mini Beadbeater 96 for the homogenization
of murine lung and thigh tissue from standard neutropenic infection models. The Mini Beadbeater 96 proved to be an effective method for homogenizing
murine lung and thigh tissues for subsequent bacterial quantitation, with results comparable to those obtained with mechanical tissue grinders.
Conclusion:
This method proved to be a practical, high throughput, time efficient, cost effective, ergonomically friendly method for tissue homogenization.
Keywords
Beadbeater; Tissue homogenization; Tissue grinders
Abbreviations
Beadbeater : Mini-Beadbeater 96; BAP: Trypticase soy agar with 5% sheep blood; MSDs: musculoskeletal disorders; STA56, ATCC29213: Staphylococcus aureus; KP462: Klebsiella pneumonia; PSA1401, PSAJJ-1-29: Pseudomonas aeruginosa
Introduction
Animal infection models are routinely used to evaluate the efficacy of antimicrobial agents. Since these models are considered predictive of clinical success, data generated from these models are used by regulatory agencies in approval processes and setting susceptibility breakpoints [1,2]. Given the general utility of these models to evaluate anti-infective therapies, their use is commonplace in antibacterial drug discovery and development research. Specific models of interest for understanding the pharmacokinetics and pharmacodynamics of antibacterials include murine lung and thigh infection models. To extract bacteria from these infected animal tissues, homogenization is required prior to quantitation of bacterial density. Although the method for tissue homogenization is not always specified in the literature, commonly used methods for a variety of endpoints include mechanical tissue grinders (generators; our standard method) [3,4], hand held Dounce homogenizers, reinforced polyethylene bags [5,6] and a Stomacher® [7,8]. Although each of these methods can effectively homogenize rodent tissues, they are labor-intensive, time consuming processes often requiring cleaning and sterilization steps between samples. Moreover, many laboratory methods are repetitive in nature and thus have the potential to cause injury to personnel over time [9,10]. Thus, any methods that Beadbeater techniques have been used to disrupt plant tissue prior to polymerase chain reaction detection of plant pathogens [11] and homogenize mouse tissue samples prior to LC-MS/MS detection of drug concentrations in these tissues [12]. Samples are efficiently homogenized by violent shaking (beating) for a specified period of time using the Beadbeater technology. Prior experience (LML) and preliminary experiments within the Center for Anti-Infective Research and Development (data not shown), determined that the Mini-Beadbeater 96 (Beadbeater) could effectively homogenize murine lung and thigh tissue required for subsequent quantitation of bacterial density. In this study we evaluated the efficiency and effectiveness of the Mini Beadbeater 96 as compared to our standard method of tissue homogenization of murine lung and thigh samples.
Materials and Methods
Bacterial strains
Two Staphylococcus aureus strains (STA56, ATCC29213), one Klebsiella pneumoniae strain (KP462) and two Pseudomonas aeruginosa strains (PSA1401, PSAJJ-1-29) were used in these studies. All strains were maintained at -80°C in skim milk and subcultured onto Trypticase soy agar with 5% sheep blood (BAP) prior to use.
Animals
Specific-pathogen free female ICR (CD-1) mice (25-30g) or Balb/c mice (17-20g) were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN). All mice were provided food and water ad libitum. This study was reviewed and approved by the Hartford Hospital Institutional Animal Care and Use Committee.
Murine Thigh Infection Model
Mice were rendered transiently neutropenic by intraperitoneal injections of cyclophosphamide (Baxter, Deerfield, IL or Sigma-Aldrich, St. Louis, MO) at 150 mg/kg and 100 mg/kg of body weight given 4 days and 1 day, respectively, prior to inoculation. Bacterial suspensions of S. aureus, K. pneumoniae and P. aeruginosa were prepared in sterile normal saline. For each bacterial isolate tested, 2 groups of mice (n=3) were inoculated intramuscularly in each thigh with 0.1 ml containing approximately 107 CFU/ml in sterile normal saline. At 2h post-infection, corresponding to our in-house procedure for the onset of therapy, 2 groups of mice (n=3) from each isolate group were euthanized by CO2 asphyxiation and cervical dislocation. Both thighs from each animal were harvested aseptically and individually homogenized by one of two methods: (1) Generator (10 x 115 mm, Pro Scientific Inc. Oxford, CT) in 14 ml polypropylene tubes (BD Falcon, Pittston, PA) with 5ml sterile normal saline to be consistent with historical homogenization volumes, or (2) Beaten in the Beadbeater (purchased from BioSpec Products, Bartlesville, OK) for 2 minutes in 5 ml polypropylene tubes (Denville Scientific, South Plainfield, NJ) with 5 ml sterile normal saline and 5 stainless steel beads (3.2 mm; BioSpec Products). Each sample was then passed through a large pore filter (280µ) to remove fibrous tissue (Interscience Bagpage+, Cole-Parmer). Serial dilutions of filtered thigh homogenates were cultured onto BAP for quantitation of bacterial density. Data outliers were identified using an interquartile range method and removed from the group mean.
Murine Lung Infection Model
Mice were rendered transiently neutropenic by intraperitoneal injections of cyclophosphamide (Baxter, Deerfield, IL or Sigma-Aldrich, St. Louis, MO) at 250 mg/kg and 100 mg/kg of body weight given 4 days and 1 day, respectively, prior to inoculation. Bacterial suspensions were prepared in sterile normal saline for K. pneumoniae and P. aeruginosa isolates or in 3% hog gastric mucin for S. aureus isolates. For each bacterial isolate tested, 4 groups of mice (n=6) were inoculated. Under 2% isofluorane anesthesia, 0.05ml of the bacterial suspension containing approximately 107 CFU/ml was instilled orally and nares were blocked. The mice aspirated the suspension into the lungs while being held vertically for approximately 60 seconds. At 2h (KP, PSA) or 3h (STA, ATCC) post-infection, 2 groups of mice (n=6) from each isolate were euthanized by CO2 asphyxiation and cervical dislocation. During studies evaluating a bead-free matrix (section 2.4.4 below), an additional two groups of animals were sacrificed 24 hours later. After euthanization, whole lung tissues from all animals were harvested aseptically and individually homogenized as described below in Sections 2.4.1 and 2.4.2. To be consistent with historical homogenization volumes, lung tissues were homogenized in 1ml of sterile normal saline. Tissue harvest time points of 2h or 3h (depending on organism) and 24h post-infection were selected to be consistent with our in-house procedures for onset of therapy and end of study, respectively.
Homogenization of Murine Lung Tissue with Generators or Beadbeater
Whole lung tissues from each animal were homogenized by one of two methods: (1) generator (7 x 120 mm, ProMulti Gen 7) in polystyrene tubes (12 x 75 mm BD Falcon, Pittston, PA) with 1ml sterile normal saline or (2) beaten in the Beadbeater for 2 minutes in 5 ml polypropylene tubes (Denville Scientific, South Plainfield, NJ) with 1 ml sterile normal saline and approximately 1 ml of zirconia/silica beads (1.0 mm; BioSpec Products). Each sample was then passed through a large pore filter (280µ) (Interscience Bagpage+, Cole-Parmer) and serial dilutions of lung homogenates were cultured onto BAP for quantitation of bacterial density. Bacterial densities outside one standard deviation were excluded from the group mean.
Homogenization of Murine Lung Tissue in the Beadbeater with and without Zirconia/Silica Beads
We wanted to streamline our tissue processing methodology even further and select one bead type for both lung and thigh tissue homogenization. While standardized use of zirconia/silica beads across models was considered, initial experiments showed that these beads were less effective in homogenizing thigh tissue and thus complete bacterial densities were not recoverable (data not shown). Thus, 3.2mm stainless steel beads currently utilized for thigh homogenization were evaluated for lung tissue homogenization. However, using the stainless steel beads to homogenize lung tissue resulted in cracked tubes and loss of sample despite modifications to the number of beads per tube, the homogenization times and the saline volume. As such, these studies evaluated the utility of murine lung tissue homogenization in the Beadbeater without using any beads. For these experiments, whole lung tissues from each animal were beaten in the Beadbeater for 2 minutes by one of two methods: (1) with or (2) without zirconia/silica beads in 5 ml polypropylene tubes (Denville Scientific, South Plainfield, NJ) with 1ml of sterile normal saline. Each sample was filtered and cultured as described above. Bacterial densities outside one standard deviation were excluded from the group mean.
Results
Murine Thigh Infection Model-Generators vs. Beadbeater
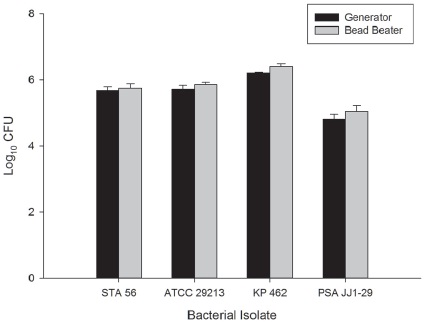
The bacterial density in murine thigh tissue harvested 2 hours post-infection from mice infected with STA56, ATCC29213, KP462 or PSAJJ1-29 and homogenized by generators was 5.68±0.11, 5.72±0.12, 6.21±0.02 and 4.81±0.15 log10 CFU, respectively and from thigh tissue homogenized by the Beadbeater was 5.75±0.14, 5.86±0.07, 6.40±0.09 and 5.05±0.17 log10 CFU, respectively (Figure 1). Upon visual inspection, the generators homogenized the thigh muscle and bone into a uniform suspension, while the Beadbeater left the bone and some muscle tissue intact. However, the resultant bacterial densities were comparable.
Figure1 Comparison of bacterial burden in murine thigh tissue 2h post-inoculation homogenized by mechanical tissue generators (black bar) or Mini Beadbeater 96 (gray bar). Bars represent mean ±SD.
Murine Lung Infection Model-Generators vs. Bead beater
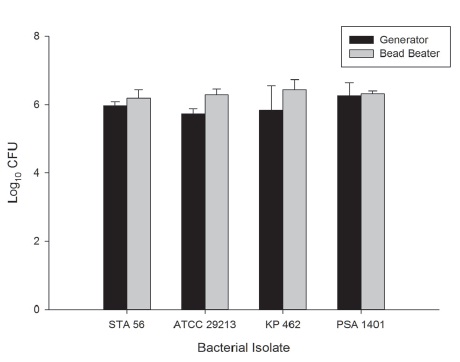
The bacterial density in murine lung tissue harvested 2 hours (KP, PSA) or 3h (STA, ATCC) post-infection from mice infected with STA56, ATCC29213, KP462 or PSA1401 and homogenized by generators was 5.97±0.11, 5.73±0.15, 5.83±0.72 and 6.25±0.39 log10 CFU, respectively and from lungs homogenized in the Beadbeater was 6.19±0.24, 6.28±0.17, 6.43±0.30 and 6.31±0.09 log10 CFU, respectively (Figure 2). Murine lung tissue processed by both generators and the Beadbeater resulted in completely homogenized, uniform suspensions with comparable bacterial densities.
Figure 2 Comparison of bacterial burden in murine lung tissue 2h (KP, PSA) or 3h (STA, ATCC) post-inoculation homogenized by mechanical tissue generators (black bar) or zirconia/silica beads in the Mini Beadbeater 96 (graybar). Bars represent mean ±SD.
Murine Lung Infection Model- Zirconia/Silica Beads vs. no Beads
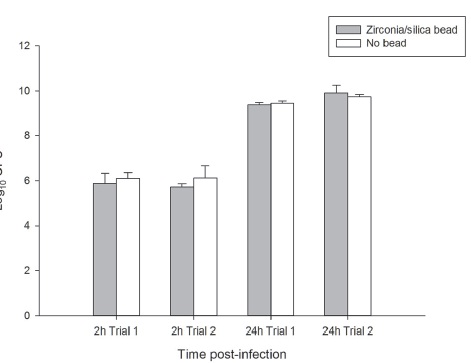
In two independent experiments, the bacterial density in murine lung tissue harvested 2 hours post-infection from mice infected with PSA1401 and homogenized with beads was 5.88±0.46 and 5.71±0.15 log10 CFU in Trial 1 and 2, respectively. The bacterial density in lung samples homogenized without beads was 6.11±0.26 and 6.13±0.52 log10 CFU in Trial 1 and 2, respectively. The bacterial density in murine lung tissue harvested at 24 hours post-infection and homogenized with beads was 9.36±0.11 and 9.91±0.33 log10 CFU in Trial 1 and 2, respectively. The bacterial density in lung samples homogenized without beads was 9.43±0.11 and 9.73±0.11 log10 CFU in Trial 1 and 2, respectively (Figure 3). Lung tissue homogenized in the Beadbeater with and without zirconia/silica beads resulted in completely homogenized, uniform suspensions with comparable bacterial densities.
Figure 3 Comparison of bacterial burden of PSA1401 in murine lung tissue 2h and 24h post-inoculation homogenized with zirconia/silica beads (gray bar) or no beads (white bar) in the Mini Beadbeater 96. Bars represent mean ±SD.
Discussion
Animal infection models are the cornerstone of antibacterial drug discovery and development. Homogenization of tissues from these models is necessary prior to quantitation of bacterial density. Although a variety of homogenization methods are routinely used, most are labor intensive, time consuming processes with the propensity towards repetitive motions.
We compared generators and the Beadbeater for homogenizing murine lung and thigh tissue by quantitating bacterial density in these tissues. Bacterial densities in infected murine thigh tissue homogenized by generators and the Beadbeater using stainless steel beads were comparable even though the generators produced uniform suspensions and the Beadbeater method left the bone and some muscle intact. Bacterial densities in infected murine lung tissue homogenized by generators and the Beadbeater using zirconia/silica beads were comparable with both methods producing completely homogenized, uniform suspensions. Bacterial densities in murine lung tissue homogenized with and without beads using the Beadbeater were comparable with both methods producing completely homogenized, uniform suspensions. Since tissue homogenization of infected murine lung and thigh tissue by both the generators and the Beadbeater resulted in similar bacterial densities, we concluded the Beadbeater method did not compromise the viability of the organisms tested. Bacterial densities in tissues from murine lung and thigh infection models can vary greatly over a multi-log range depending on the inoculum, time of sampling post-infection and efficacy of the therapeutic agents tested. If the homogenization methods were different we would expect to see greater differences in CFU at 2 or 3 hours post-infection where bacterial burdens are lower. However, bacterial densities were comparable at 2 or 3 hours post-infection as well as at 24h where bacterial densities were higher, suggesting these homogenization methods are comparable.
We noted several advantages to using the Beadbeater rather than generators to homogenize murine tissues. Previously we homogenized tissue samples individually using generators that required cleaning (water and ethanol rinses) between samples. Since we typically homogenize 150-200 tissue samples per experiment, the ability to homogenize multiple samples in a short time frame without additional cleaning steps or preparation of water and ethanol rinse tubes resulted in a considerable reduction in overall preparation and tissue processing time. We now routinely homogenize 12 tissue samples in 2 minutes with the Beadbeater. While use of the Beadbeater, even with differing beads for lung and thigh infection models was advantageous, further refinement of the tissue homogenization process by eliminating beads entirely for lung tissue homogenization resulted in reduced cost of materials and labor.
Many laboratory procedures are repetitive in nature such as capping and uncapping tubes, holding tubes, pipetting and vortexing samples. These repetitive procedures have the potential to cause musculoskeletal disorders (MSDs) and have been identified by OSHA as ergonomic problems [9,10]. By using the Beadbeater, we can eliminate the preparation and use of the tubes that are needed to clean and rinse the generators, eliminate capping and recapping the tubes associated with the cleaning and homogenization process, and eliminate the exposure to vibration associated with the use of generators. These translated into savings in terms of homogenization time, cost of supplies and associated labor and reduced the potential exposure of laboratory personnel to MSDs.
The studies described herein found Beadbeater technology to be an effective method for homogenizing murine lung and thigh tissue for subsequent bacterial quantitation, with results comparable to our historical laboratory standard, mechanical generators. Based on these findings, our lab has adopted this as a practical, cost effective, high throughput, labor and time efficient, ergonomically friendly method for homogenizing murine lung and thigh tissues.
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
LML, JT-R, JLC and DPN contributed to the conception and design of this study, acquisition and analysis of the data, drafting of the manuscript and critical revision. All authors have read and approved the final manuscript.
Acknowledgements
The authors wish to thank Mary Anne Banevicius, Debora Santini, Pamela Tessier and Lindsay Tuttle for technical assistance with the animal experiments. We also wish to thank Glenn Gibson (Pfizer) for initial experiments assessing the utility of the Beadbeater for murine lung and thigh tissue homogenization. Funding for the conduct of these experiments was provided internally by the Center for Anti-Infective Research and Development.
References
- Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, et al. (2007) Pharmacokinetics-Pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin Infect Dis 44: 79-86.
- Craig WA (1998) Pharmcokinetic/Pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin Infect Dis 26: 1-12.
- Banevicius MA, Kaplan N, Hafkin B, Nicolau DP (2013) Pharmacokinetics, pharmacodynamics and efficacy of novel FabI inhibitor AFN-1252 against MSSA and MRSA in the murine thigh infection model. J Chemother 25: 26-31.
- Koomanachai P, Crandon JL, Banevicius MA, Peng L, Nicolau DP (2009) Pharmacodynamic Profile of Tigecycline against methicillin-resistant Staphylococcus aureus in an experimental pneumonia model. Antimicrob Agents Chemother 53: 5060-5063.
- Lepak AJ, Marchillo K, VanHecker J, Andes DR (2013) Isavuconazole (BAL4815) pharmacodynamic target determination in an in vivo murine model of invasive pulmonary aspergillosis against wild-type and cyp51 mutant isolates of Aspergillus fumigatus. Antimicrob Agents Chemother 57: 6284-6289.
- Walsh TJ, McEntee C, Dixon DM (1987) Tissue homogenization with sterile reinforced polyethylene bags for quantitative culture of Candida albicans. J Clin Micro 25: 931-932.
- Marra A, Lawson S, Asundi JS, Brigham D, Hromockyj AE (2002) In vivo characterization of the psa genes from Streptococcus pneumoniaein multiple models of infection. Microbiology 148: 1483-1491.
- Baliban SM, Michael A, Shammassian B, Mudakha S, Khan AS, et al. (2014) An optimized, synthetic DNA vaccine encoding the Toxin A and Toxin B receptor binding domains of Clostridium difficile induces protective antibody responsesin vivo. Infect Immun 82: 4080-4091.
- https://www.osha.gov/Publications/laboratory/OSHAfactsheet-laboratory-safety-ergonomics.pdf
- https://www.osha.gov/SLTC/ergonomics/identifyprobs.html
- Vincelli P, Amsden, B (2013) Comparison of tissue-disruption methods for PCR-based detection of plant pathogens. Plant Dis 97: 363-368.
- Liang X, Ubhayakar S, Liederer BM, Dean B, Ran-Ran Qin A, et al. (2011) Evaluation of homogenization techniques for the preparation of mouse tissue samples to support drug discovery. Bioanalysis 3: 1923-1933.