Research Article
S. Mehran Hosseini, MD. PhD1*, Alireza Maleki2, and S. Mohsen Hosseininejad2
1Department of Physiology, Golestan University of Medical Sciences, Gorgan, Iran
2Medical Student at Golestan University of Medical Sciences
Corresponding author
Dr. S. Mehran Hosseini, MD, PhD, Department of Physiology, Golestan University of Medical Sciences, P.O. Box: 49175-553, Gorgan, Iran, Tel: +9113736634; fax: +981714440225; E-mail: hosseini@goums.ac.ir
Received Date: 05th August 2014
Accepted Date: 29th September 2014
Published Date: 06th October 2014
Citation
Hosseini SM (2014) Cardiac Autonomic Neuropathy and Two Insulin Resistance Indices. Enliven: Clin Cardiol Res 1(3): 006.
Copyright
@ 2014 Dr. S. Mehran Hosseini. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background and objectives: Cardiac autonomic neuropathy (CAN) is a common and poor prognosis complication of diabetes. There are documents regarding the causative role of autonomic nervous system in the pathogenesis of insulin resistance (IR). As a predisposing factor for diabetes and metabolic syndrome, the IR by itself is also associated with increased risk of cardiovascular disease. Very limited studies concern the relation of IR with CAN. Therefore the values of the homeostasis model assessment of insulin resistance (HOMA-IR) and the triglyceride-glucose (TyG) indices were studied in diabetic patients with CAN.
Methods: This descriptive cross sectional study was carried on 70 volunteer diabetic’s participants in endocrine clinic of Fifth AZARE hospital in Gorgan that full fit the inclusion criteria. The following tests were used for diagnosis of CAN: resting heart rate, orthostatic challenge tests and corrected QT interval. Two IR indices were calculated for patients based on fasting levels of glucose, insulin and triglyceride.
Results: The mean±SD for age and weight were 55.19±9.79 years and 76.1±10.22 kilograms. The CAN was diagnosed in 54 cases (85.3%). The mean±SD for TyG and HOMA-IR were 10.11±0.69 and 1.6±0.98 respectively. The TyG but not the HOMA-IR index was more than the considered cutoff in all cases including CAN negatives. The difference of HOMA-IR index in CAN positive and CAN negative subjects was not significant (P value = 0.059).
Conclusion: The changes of TyG index seem to be much more than HOMA-IR in diabetics with CAN and may be considered to have screening potential for CAN.
Keywords
Cardiac Autonomic Neuropathy; Insulin resistance; TyG; HOMA
Introduction
A common and poor prognosis complication of diabetes is cardiac autonomic neuropathy (CAN) [1-3]. The sensory and the motor functions of both sympathetic and parasympathetic nerves are involved in CAN [4,5]. There are also documents regarding the causative role of autonomic nervous system on pathogenesis of insulin resistance IR [6,7]. IR is associated with increased risk of cardiovascular disease per se [8]. Both the peripheral and the hepatic forms of IR have important role in pathologic glucose homeostasis, diabetes and metabolic syndrome. The severity of IR is expressed by many numerical indices [9]. This quantification provides more information about the underlying metabolic state and may potentially be linked to some underlying complications including the CAN. The HOMA-IR is the well-known and most popular index that has good correlation with the gold standard for IR assessment e.g. the hyperinsulinemic euglycemic glucose clamp test. Recently a product of triglyceride and glucose, the TyG index is offered as a new tool for IR assessment [10-12]. Two items for metabolic syndrome identification including the hypertriglyceridemia and the elevated fasting glucose are directly reflected in TyG index. It is hypothesized that monitoring of IR indices may be useful in clinical work up of CAN. But according to our limited knowledge and our accessible literature review there are very few reports regarding this topic. Therefore the values of HOMA-IR and TyG indices were studied in a group of diabetic patients with CAN.
Methods
This descriptive cross sectional study was carried on 70 volunteer diabetic?s type II participants in endocrine clinic of Fifth AZARE hospitals in Gorgan that full fit the inclusion criteria. The subjects that had completed all autonomic and blood tests in one session were 54 and 49 persons for TyG and HOMA-IR respectively. The following tests were used for diagnosis of CAN: resting heart rate, orthostatic changes in heart rate and blood pressure following active standing and corrected QT interval [13]. The patients were categorized in 3 groups: 1 or without CAN when all tests were normal, 2 or borderline when at least one abnormal test was exist and 3 or CAN positive in cases who had two or more abnormal tests [13]. Fasting level of glucose, insulin and triglyceride were determined and used for
HOMA and TyG index calculations according to the following formula [10,11]:
TyG index= Ln [(Fasting triglyceride, mg/dL ) × ( Fasting glucose, mg/dL)/2]
HOMA-IR index= Fasting glucose (mmol/L) ×fasting insulin (mU/L)/22.5
All data were expressed as mean±SD and P values less than 0.05 was set for statistical significance using SPSS 16.
Results
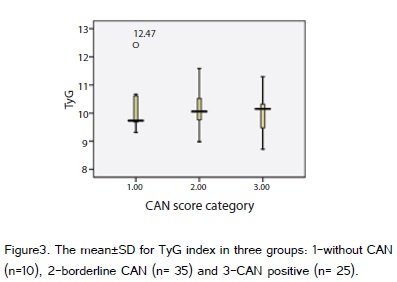
The mean±SD of age and weight were 55.19±9.79 years and 76.1±10.22 kilograms. There were 48 female (68.6%). The basic characteristics of patients were shown in table 1. According to autonomic test results the final scoring was as follow: 10 patients (14.3%) had 1 score e.g. without CAN, 35 patients (50%) had 2 score and were considered borderline and 25 patients (35.7%) had 3 score which indicated the CAN. The CAN was diagnosed in 60 cases (85.7%). The Chi Square (?2) test did not show any significant differences regarding gender and other basic characters between three groups e.g.: 1-without CAN, 2-borderline CAN and 3-CAN positive.
| min | max | mean | SD | |
| Age (years) | 38 | 83 | 55.19 | 9.79 |
| Height (cm) | 147 | 184 | 161.36 | 8.27 |
| Weight (Kg) | 56 | 97 | 76.1 | 10.22 |
| BMI (Kg.m-2) | 21.45 | 39.35 | 29.3 | 3.94 |
| Diabetes duration (years)* | 2 | 25 | 9.03 | 5.54 |
*Based on patients self reports.
Table1. The basic characteristics of type II diabetic patients (n=70)
The male/female distribution in three CAN groups was shown in figures 1.
For all cases the mean±SD of TyG and HOMA-IR were 10.11±0.69 and 1.6±0.98 respectively. These parameters were shown separately for three groups in figure 2 and 3. Since the normal and native age-and sex-dependent ranges for these indices were not well documented, the cutoff values for diagnosis of IR based on TyG and HOMA-IR indices were roughly considered 9 and 2.5 respectively [10,15]. The TyG was more than the considered cutoff in all cases including CAN negatives; however only 12 cases (24.5%) had passed the cutoff for HOMA-IR index. The difference of HOMA-IR index in CAN positive and CAN negative subjects was not significant (P value = 0.059). When considering the diabetes duration there was significant differences among three groups (p=0.041). The systolic blood pressure changes in comparison to other orthostatic tests showed the maximum correlation with CAN scores (r=0.509).
Discussion
This study demonstrates the dissimilarity of two IR indexes in CAN. The non-significant differences of HOMA-IR index in a CAN study between diabetics and healthy normal controls had been reported previously and the limited sample size was expressed reason [16]. Our data was also show a similar result for HOMA-IR index between groups, but in all cases it was also less than the cut point. However the TyG index was more than the cut point in all cases including CAN negatives. The TyG index in borderline and CAN positives was more than the CAN negatives. These observations may be related to ethnicity, genetic polymorphism or dietary habits that cause higher prevalence of metabolic syndrome in our population [17-20]. The early diagnosis of subclinical CAN has prognostic values and CAN screening is recommended for type II diabetics whenever of its diagnosis even in asymptomatic cases [21]. Literature data regarding the screening for CAN is mainly limited to autonomic reflexes. Very limited studies are looking for other screening tools or markers for CAN. Recently the association of adipose tissue inflammatory markers with CAN was reported [22,23]. The association of increased HOMA-IR with impaired myocardial perfusion in prediabetics adult may also be linked to CAN [24]. The obesity and IR are interrelated and both of them are known as predisposing factor for diabetes. TyG index includes two out of five of metabolic syndrome conditions: the elevated fasting plasma glucose and the high serum triglycerides levels. The sensitivity and specificity of TyG index can compete with other markers for IR [10,12] Unlike the homeostasis model assessment (HOMA) and quantitative insulin sensitivity check index (QUICKI), insulin is not included in TyG index. This simplicity has practical outcomes such as accessibility and less cost that may be very important in low-income populations at risk for metabolic syndrome and diabetes [11]. The estimated prevalence of CAN in studied sample was higher than other reports. This may be related to different diagnostic criteria or genetic factors [25-29], however the unawareness of patients about the manifestation of CAN was also especially obvious in the course of study. The most important risk factor for CAN in studied sample is more than 10 years diabetic history. Although this descriptive pilot cannot show significant difference of TyG index between groups but there were more abnormally high values in comparison with HOMA-IR index. Several limitations of this study must be considered: small sample size, using the non native cut points for IR indices and the lack of complete references for age- and sex- specific values for some autonomic tests. The sensitivity and specificity of TyG and HOMA-IR indices were also not considered in this pilot. Further studies are required to evaluate the use of TyG index in CAN.