Research Article
Authors:
Shahin Asadi*1, Mahsa Jamali2, Hamideh Mohammadzadeh2, Vida Vahdani Kia2, Rana Bagheri2 and Roya Pourjafar2
1 Master of molecular genetics, Director of the Division of Medical Genetics and Molecular Research, Iran
2 Master of molecular genetics, Division of Medical Genetics and Molecular Research, Iran
Corresponding author
Shahin Asadi, Molecular Genetics-IRAN-TABRIZ, Director of the Division of Medical Genetics and Molecular Research, Iran, Tel:+989379923364, E-mail: shahin.asadi1985@gmail.com
Received Date: 13 October 2017; Accepted Date: 14 November 2017; Published Date: 20 November 2017
Citation
Shahin A, Mahsa J, Hamideh M, Vahdani K, Rana B, et al. (2017) Assessment of Genetic Mutations in Patients with Autism and Schizophrenia Nerve Cells in Tabriz, Iran 2017. Enliven: J Genet Mol Cell Biol 4(2): 001.
Copyright
@ 2017 Shahin Asadi. This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, that permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
In this study we have analyzed 100 people. 40 patients Autism disease and 60 persons control group.The gene polymorphisme 5-HTTLPR analyzed in terms of genetic mutations made. In this study, people who have genetic mutations were targeted, with nervous disorders, Autism disease. In fact, of all people with Autism disease. 40 patients Autism disease had a genetic mutations in the gene polymorphisme 5-HTTLPR Autism disease. Any genetic mutations in the target genes control group, did not show. The aim of this review is to summarize the key findings from genetic and epidemiological research, which show that autism is a complex disorder resulting from the combination of genetic and environmental factors. Remarkable advances in the knowledge of genetic causes of autism have resulted from the great efforts made in the field of genetics. The identification of specific alleles contributing to the autism spectrum has supplied important pieces for the autism puzzle. However, many questions remain unanswered, and new questions are raised by recent results. Moreover, given the amount of evidence supporting a significant contribution of environmental factors to autism risk, it is now clear that the search for environmental factors should be reinforced. One aspect of this search that has been neglected so far is the study of interactions between genes and environmental factors.
Introduction
The heritability of autism is the proportion of autism that can be explained by genetic variation; if the heritability of a condition is high, then the condition is considered to be primarily genetic. Autism has a strong genetic basis, although the genetics of autism is complex and it is unclear whether autism spectrum disorder (ASD) is explained more by multi gene interactions or by rare mutations with major effects [1].
Early studies of twins estimated the heritability of autism to be more than 90%--meaning that 90% of the differences between autistic and non-autistic individuals was due to genetics [2]. This may be an overestimate: new twin data and models with structural genetic variation are needed [3]. When only one identical twin is autistic, the other often has learning or social disabilities [4]. For adult siblings, the risk of having one or more features of the broader autism phenotype might be as high as 30%, [5] much higher than the risk in controls [6].
Genetic linkage analysis has been inconclusive; many association analyses have had inadequate power [3]. For each autistic individual, mutations in more than one gene may be implicated. Mutations in different sets of genes may be involved in different autistic individuals. There may be significant interactions among mutations in several genes, or between the environment and mutated genes. By identifying genetic markers inherited with autism in family studies, numerous candidate genes have been located, most of which encode proteins involved in neural development and function [7,8]. However, for most of the candidate genes, the actual mutations that increase the risk for autism have not been identified. Typically, autism cannot be traced to a Mendeli an (single-gene) mutation or to single chromosome abnormalities such as fragile X syndrome or 22q13 deletion syndrome [9,10].
The large number of autistic individuals with unaffected family members may result from copy number variations (CNVs)—spontaneous alterations in the genetic material during meiosis that delete or duplicate genetic material [11,12]. Sporadic (non-inherited) cases have been examined to identify candidate genetic loci involved in autism. A substantial fraction of autism may be highly heritable but not inherited: that is, the mutation that causes the autism is not present in the parental genome [13].
Although the fraction of autism traceable to a genetic cause may grow to 30–40% as the resolution of array CGH improves,[13] several results in this area have been described incautiously, possibly misleading the public into thinking that a large proportion of autism is caused by CNVs and is detectable via array CGH, or that detecting CNVs is tantamount to a genetic diagnosis [14]. The Autism Genome Project database contains genetic linkage and CNV data that connect autism to genetic loci and suggest that every human chromosome may be involved [15]. It may be that using autism-related subpheno types instead of the diagnosis of autism per se may be more useful in identifying susceptible loci [16].
Twin studies are a helpful tool in determining the heritability of disorders and human traits in general. They involve determining concordance of characteristics between identical (monozygotic or MZ) twins and between fraternal (dizygotic or DZ) twins. Possible problems of twin studies are: (1) errors in diagnosis of monozygo city, and (2) the assumption that social environment sharing by DZ twins is equivalent to that of MZ twins.
A condition that is environmentally caused without genetic involvement would yield a concordance for MZ twins equal to the concordance found for DZ twins. In contrast, a condition that is completely genetic in origin would theoretically yield a concordance of 100% for MZ pairs and usually much less for DZ pairs depending on factors such as the number of genes involved and assortative mating.
An example of a condition that appears to have very little if any genetic influence is irritable bowel syndrome (IBS), with a concordance of 28% vs. 27% for MZ and DZ pairs respectively [17]. An example of a human characteristics that is extremely heritable is eye color, with a concordance of 98% for MZ pairs and 7–49% for DZ pairs depending on age [18].
Identical twin studies put autism's heritability in a range between 36% and 95.7%, with concordance for a broader phenotype usually found at the higher end of the range [19]. Autism concordance in siblings and fraternal twins is anywhere between 0 and 23.5%. This is more likely 2–4% for classic autism and 10–20% for a broader spectrum. Assuming a general-population prevalence of 0.1%, the risk of classic autism in siblings is 20- to 40-fold that of the general population.
Notable twin studies have attempted to shed light on the heritability of autism.
A small scale study in 1977 was the first of its kind to look into the heritability of autism. It involved 10 DZ and 11 MZ pairs in which at least one twin in each pair showed infantile autism. It found a concordance of 36% in MZ twins compared to 0% for DZ twins. Concordance of "cognitive abnormalities" was 82% in MZ pairs and 10% for DZ pairs. In 12 of the 17 pairs discordant for autism, a biological hazard was believed to be associated with the condition [20].
A 1979 case report discussed a pair of identical twins concordant for autism. The twins developed similarly until the age of 4, when one of them spontaneously improved. The other twin, who had suffered infrequent seizures, remained autistic. The report noted that genetic factors were not "all important" in the development of the twins [21].
In 1985, a study of twins enrolled with the UCLA Registry for Genetic Studies found a concordance of 95.7% for autism in 23 pairs of MZ twins, and 23.5% for 17 DZ twins [22].
In a 1989 study, Nordic countries were screened for cases of autism. Eleven pairs of MZ twins and 10 of DZ twins were examined. Concordance of autism was found to be 91% in MZ and 0% in DZ pairs. The concordances for "cognitive disorder" were 91% and 30% respectively. In most of the pairs discordant for autism, the autistic twin had more perinatal stress [23].
A British twin sample was reexamined in 1995 and a 60% concordance was found for autism in MZ twins vs. 0% concordance for DZ. It also found 92% concordance for a broader spectrum in MZ vs. 10% for DZ. The study concluded that "obstetric hazards usually appear to be consequences of genetically influenced abnormal development, rather than independent aetiological factors” [24].
A 1999 study looked at social cognitive skills in general-population children and adolescents. It found "poorer social cognition in males", and a heritability of 0.68 with higher genetic influence in younger twins [25].
In 2000, a study looked at reciprocal social behavior in general-population identical twins. It found a concordance of 73% for MZ, i.e. "highly heritable", and 37% for DZ pairs [26].
A 2004 study looked at 16 MZ twins and found a concordance of 43.75% for "strictly defined autism". Neuroanatomical differences (discordant cerebellar white and grey matter volumes) between discordant twins were found. The abstract notes that in previous studies 75% of the non-autistic twins displayed the broader phenotype [27].
Another 2004 study examined whether the characteristic symptoms of autism (impaired social interaction, communication deficits, and repetitive behaviors) show decreased variance of symptoms among monozygotic twins compared to siblings in a sample of 16 families. The study demonstrated significant aggregation of symptoms in twins. It also concluded that "the levels of clinical features seen in autism may be a result of mainly independent genetic traits” [28].
An English twin study in 2006 found high heritability for autistic traits in a large group of 3,400 pairs of twins [29].
One critic of the pre-2006 twin studies said that they were too small and their results can be plausibly explained on non-genetic grounds [30] .
Materials and Methods
In this study, 100 patients with autism disease and 150 persons control group were studied. Peripheral blood samples from patients and parents with written permission control was prepared. After separation of serum, using Real Time-PCR technique; tRNA molecules were collected. To isolate Neuroglial cells erythrocytes were precipitated from hydroxyethyl starch (HES) was used. At this stage, HES solution in ratio of 1to5with the peripheral blood of patients and controls were mixed. After 60 minutes of incubation at room temperature, the supernatant was removed and centrifuged for 14 min at 400 Gera. The cell sediment with PBS (phosphate buffered saline), pipetazh and slowly soluble carbohydrate ratio of 1to2 on ficole (Ficol) was poured in the 480G was centrifuged for 34 minutes.Mono nuclear Neuroglial cells also are included, has a lower density than ficole and soon which they are based.The remaining erythrocytes has a molecular weight greater than ficoleand deposited in test tubes.
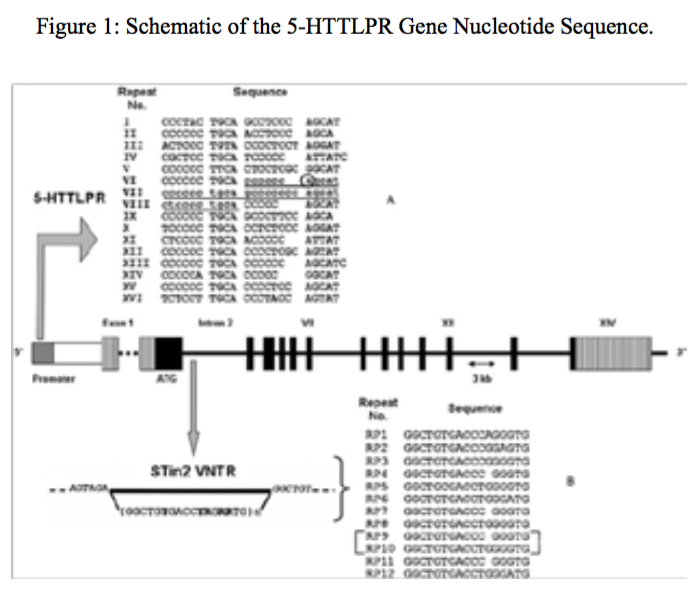
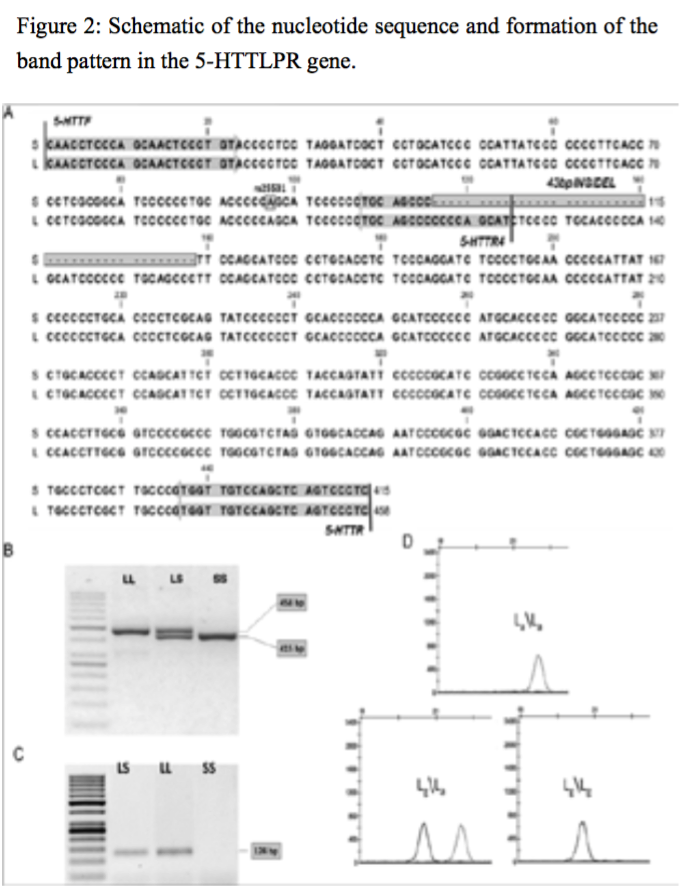
The supernatant, which contained the mono nuclear cells was removed, and the 400 Gera was centrifuged for 12 minutes. Finally, the sediment cell, the antibody and Neuroglial cells was added after 34 minutes incubation at 5°C, the cell mixture was passed from pillar LSMACS. Then the cells were washed with PBS and attached to the column LSMACSS Pam Stem cell culture medium containing the transcription gene 5-HTTLPR,and were kept (Figure1, Figure 2).
Figure 1:Schematic of the 5-HTTLPR Gene Nucleotide Sequence.
Figure 2:Schematic of the nucleotide sequence and formation of the band pattern in the 5-HTTLPR gene.
Results
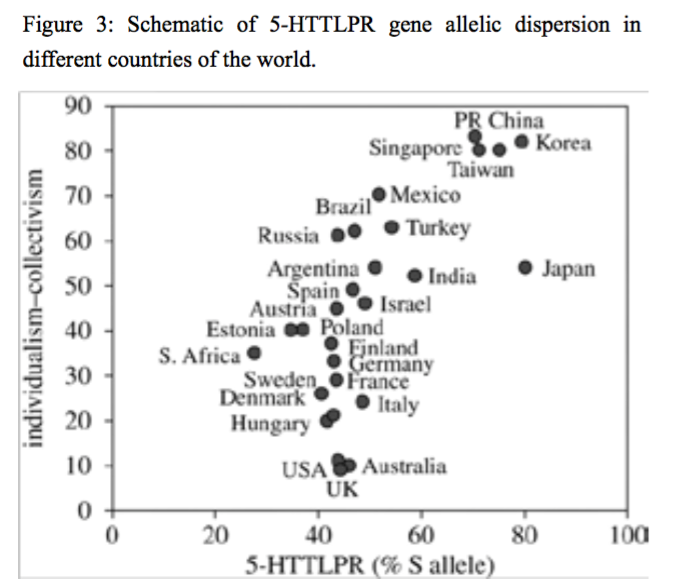
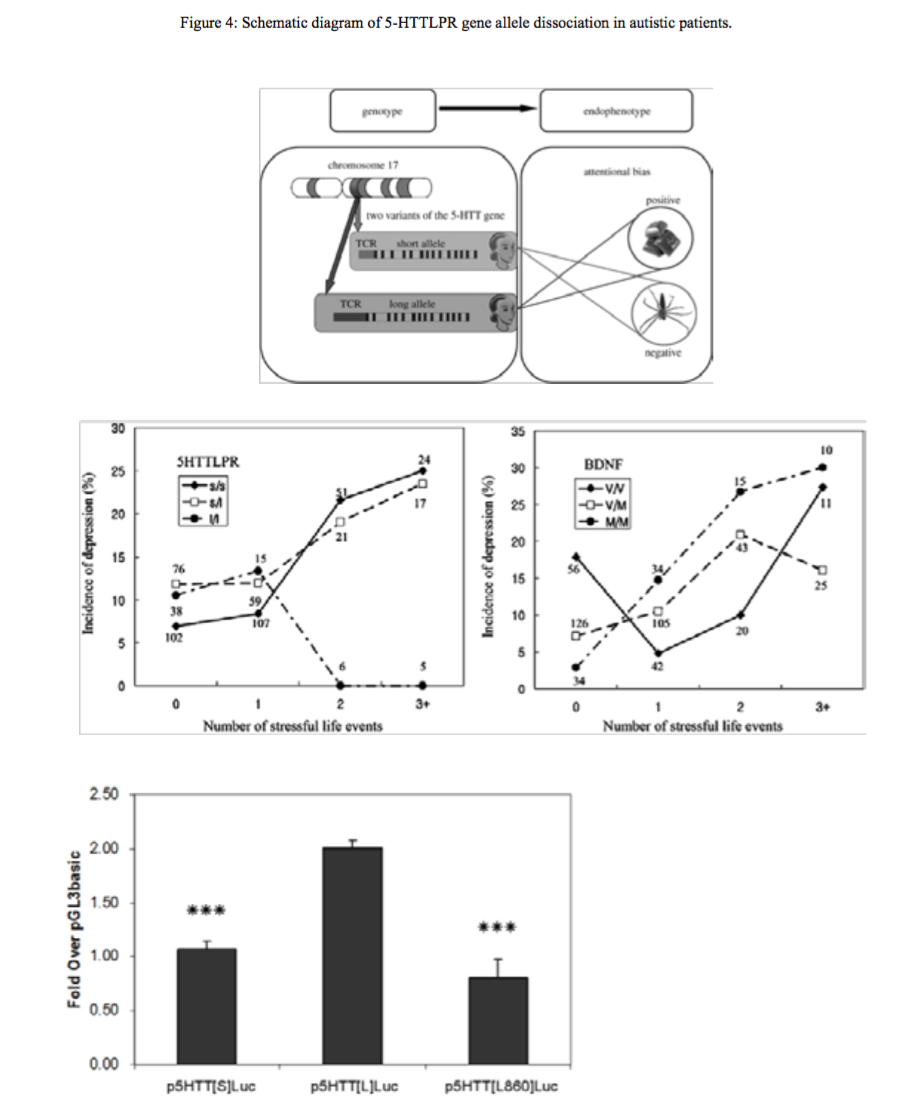
Insert figures here (Figure 3, Figure 4, Figure 5, Figure 6).
Figure 3:Schematic of 5-HTTLPR gene allelic dispersion in different countries of the world.
Figure 4:Schematic diagram of 5-HTTLPR gene allele dissociation in autistic patients.
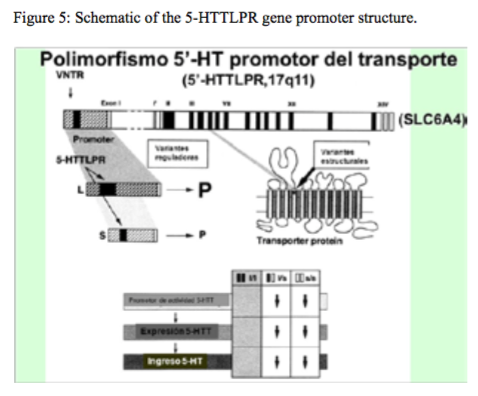
Figure 5:Schematic of the 5-HTTLPR gene promoter structure.
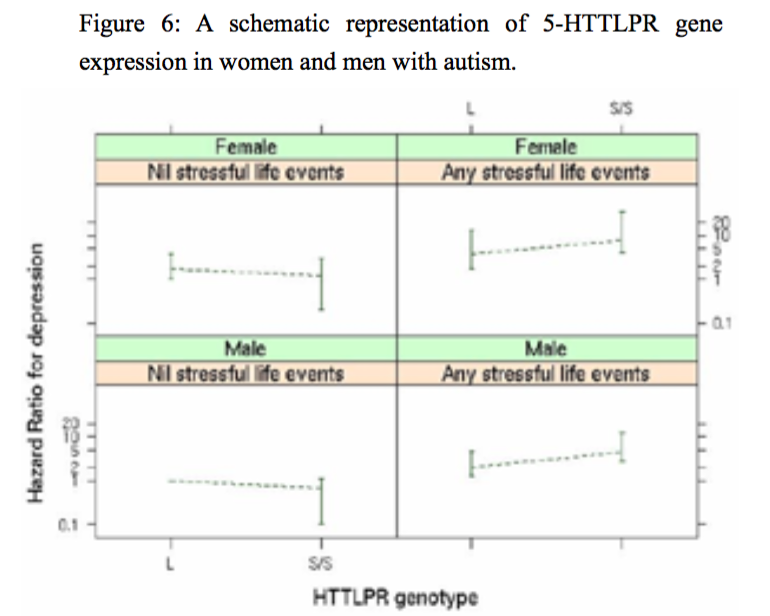
Figure 6:A schematic representation of 5-HTTLPR gene expression in women and men with autism.
Conclusion
Today, mental disorders such as autism and schizophrenia as a disease pandemic in human beings is for all regions of the world and of all races. In all societies, children with autism and schizophrenia inception of the disorder as a potential in nerve cells themselves are stored. Currently, autism and schizophrenia do not make any decisive factor to induce the disease. But mutation in a variety of autism and schizophrenia susceptibility genes in the nervous system is very important. In this study, a variety of genetic mutations to autism and schizophrenia was analyzed. The results of this study suggest that mutations in genes that affect nerve cells and exposure to environmental factors, can be very important for autism and schizophrenia. So nothing more significant than the DNA molecules to induce disease will be. And disturbance or irregularity in the sequence of DNA molecules can be the most important factor for diseases of talent.
References
- Sykes NH, Lamb JA (2007) Autism: the quest for the genes. Expert Rev Mol Med 9: 1-15.
- Le Couteur A, Bailey A, Goode S, Pickles A, Robertson S, et al. (1996) A broader phenotype of autism: the clinical spectrum in twins. J Child Psychol Psychiatry 37: 785-801.
- Folstein SE, Rosen-Sheidley B (2001) Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet 2: 943-955.
- Bolton P, Macdonald H, Pickles A, Rios P, Goode SA, et al. (1994) A case-control family history study of autism. J Child Psychol Psychiatry 35: 877-900.
- Persico AM, Bourgeron T (2006) Searching for ways out of the autism maze: genetic, epigenetic and environmental clues. Trends Neurosci 29: 349-358.
- Yang MS, Gill M (2007) A review of gene linkage, association and expression studies in autism and an assessment of convergent evidence. Int J Dev Neurosci 25: 69-85.
- Cohen D, Pichard N, Tordjman S, Baumann C, Burglen L et al. (2005) Specific genetic disorders and autism: clinical contribution towards their identification. J Autism Dev Disord 35: 103-116.
- Beaudet AL (2007) Autism: highly heritable but not inherited. Nat Med 13: 534-536.
- Cook EH, Scherer SW (2008) Copy-number variations associated with neuropsychiatric conditions. Nature 455: 919-923
- (2007) The Autism Genome Project Consortium. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet 39: 319-328.
- Bito LZ, Matheny A, Cruickshanks KJ, Nondahl DM, Carino OB (1997) Eye color changes past early childhood. The Louisville Twin Study. Arch Ophthalmol 115: 659-663.
- Folstein S, Rutter M (1977) Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry 18: 297-321.
- Mohammed I, Cherkas LF, Riley SA, Spector TD, Trudgill NJ (2005) Genetic influences in irritable bowel syndrome: a twin study. Am J Gastroenterol 100: 1340-1344.
- Wessels WH, Pompe van Meerdervoort M (1979) Monozygotic twins with early infantile autism. A case report. S Afar Med J 55: 955-957.
- . Ritvo ER, Freeman BJ, Mason-Brothers A, Mo A, Ritvo AM (1985) Concordance for the syndrome of autism in 40 pairs of afflicted twins. Am J Psychiatry 142: 74-77.
- Steffenburg S, Gillberg C, Hellgren L, Andersson L, Gillberg IC et al. (1989) A twin study of autism in Denmark, Finland, Iceland, Norway and Sweden. J Child Psychol Psychiatry 30: 405-416.
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E et al. (1995) Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25: 63-77.
- Scourfield J, Martin N, Lewis G, McGuffin P (1999) Heritability of social cognitive skills in children and adolescents. Br J Psychiatry 175:559-564
- Constantino JN, Todd RD (2000) Genetic structure of reciprocal social behavior. Am J Psychiatry 157:2043-2045.
- Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D et al. (2004) Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. Am J Psychiatry 161: 539-546.
- Kolevzon A, Smith CJ, Schmeidler J, Buxbaum JD, Silverman JM (2004) Familial symptom domains in monozygotic siblings with autism. Am J Med Genet B Neuropsychiatr Genet 129: 76-81.
- Ronald A, Happé F, Bolton P, Butcher LM, Price TS et al. Genetic heterogeneity between the three components of the autism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry 45:691-699.
- Hughes C, Plumet MH, Leboyer M (1999) Towards a cognitive phenotype for autism: increased prevalence of executive dysfunction and superior spatial span amongst siblings of children with autism. J Child Psychol Psychiatry 40:705-718.
- Lauritsen MB, Pedersen CB, Mortensen PB (2005) Effects of familial risk factors and place of birth on the risk of autism: a nationwide register-based study. J Child Psychol Psychiatry 46:963-971.
- Piven J, Wzorek M, Landa R, Lainhart J, Bolton P et al. (1994) Personality characteristics of the parents of autistic individuals. Psychol Med 24: 783-795.
- Wheelwright S, Baron-Cohen S (2001) The link between autism and skills such as engineering, maths, physics and computing: a reply to Jarrold and Routh. Autism 5: 223-237.
- Happé F, Briskman J, Frith U (2001) Exploring the cognitive phenotype of autism: weak "central coherence" in parents and siblings of children with autism: I. Experimental tests. J Child Psychol Psychiatry 42: 299-307.
- Abramson RK, Ravan SA, Wright HH, Wieduwilt K, Wolpert CM et al. (2005) The relationship between restrictive and repetitive behaviors in individuals with autism and obsessive compulsive symptoms in parents. Child Psychiatry Hum Dev 36: 155-165.
- Constantino JN, Todd RD (2005) Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry 57: 655-660.
- Ghaziuddin M (2005) A family history study of Asperger syndrome. J Autism Dev Disord 35: 177-182.