Research Article
Authors:
Rafiqul Islam1*, Sumit Kar1, and Clarinda Islam2
1Celerion, Inc., 621 Rose St, Lincoln, NE 68502 USA
2Somru BioScience, Inc., 621 Rose St, Lincoln, NE 68502 USA
Corresponding author
Rafiqul Islam, Senior Director, Celerion, 621 Rose St, Lincoln, NE 68502 USA, Tel: +1 402 437 4704, Fax: +1 402 939 0428 E-mail: Rafiqul.Islam@celerion.com
Received Date: 21 March 2018; Accepted Date: 04 April 2018; Published Date: 10 April 2018
Citation
Islam R, Kar S, and Islam C (2018) Accuracy Matters: Enzymatic Assays for Creatinine Biomarker Measurement during Drug Development. Enliven: Bio Anal Techniques 5(1): 001.
Copyright
@ 2018 Rafiqul Islam This is an Open Access article published and distributed under the terms of the Creative Commons Attribution License, that permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Glomerular filtration rate and staging of chronic kidney disease (CKD) is typically assessed by measuring the endogenous concentrations of creatinine. Bioanalytical use of creatinine is also increasing for drug development leading to greater FDA scrutiny. Creatinine is commonly measured by the rapid and inexpensive colorimetric Jaffe reaction. However, the Jaffe reaction is notoriously inaccurate and very small shifts in creatinine cause large miscalculations in CKD staging. While attempts have been made to improve Jaffe reaction assays, an enzymatic assay for creatinine is more accurate and more stable. We review substances found in many common disease states that create chromo gens that interfere is the Jaffe reaction but do not affect the enzymatic assay. This includes cephalosporin antibiotics, glucose, and bilirubin. We also provide a framework for modifying creatinine commercial kits for drug development studies. Most creatinine kits, whether using Jaffe or enzymatic chemistry, are based on the Clinical and Laboratory Standard Institute (CLSI) validation approach, which is designed to distinguish diseased from healthy, is not suitable for use in drug development. The benefits of enzymatic creatinine assays have been demonstrated for over a decade yet the Jaffe reaction continues to be the primary method of measurement demonstrating the need for continued education and nuanced recommendations. For clinical lab use, we highlight a two-phase reflexive method that balances cost and accuracy. For critical decisions during a drug development study, the data strongly supports the use of the enzymatic creatinine assay in combination with bioanalytical method validation for the high accuracy needed in these settings.
Introduction
The real world clinical and drug development applications of biomarkers discovered through over a decade of basic science biomarker research have been limited. Accordingly, regulatory bodies such as the FDA have started biomarker qualification programs to ensure biomarkers are appropriately used during clinical studies [1]. A primary objective of this process is to reduce ?measurement errors that could result in biases and affect the biomarker?s predictive accuracy thus [limiting] its utility as a valuable drug development tool? [2]. In regard to endogenous renal biomarkers, this program has resulted in the qualification of both novel and existing renal biomarkers, including creatinine, for monitoring kidney function in drug development [3-4]. In this article, we evaluate the analytical performance of creatinine assays to review guidelines for its use in drug development.
Accurate creatinine measurements are critical for efforts to improve the diagnosis and treatment of CKD and other bioanalytical uses of creatinine. The benefits of enzymatic creatinine assays (ECr) over standard kinetic Jaffe assays (JCr) have been argued for over 10 years [5-6]. Despite these recommendations JCr assays are still commonly used. In a recent study at a German hospital center, 15% of patients diagnosed with acute kidney injury were detected with JCr assays alone [7]. Use of JCr assays alone are estimated to result in a 4% miscalculation rate of serum creatinine levels overall [8]. This error rate may be acceptable in a clinical lab screening setting due to the increased cost of ECr assays and the intrinsic biological variability of creatinine concentrations. However, studies have shown that minimal shifts in creatinine results can cause major alterations in GFR calculations and patient classification [6-9]. In drug development, precise determination of kidney function with biomarkers with the lowest analytical and biological variability is necessary to determine therapeutic efficacy and obtain approval by regulatory bodies.
Accurate creatinine measurements are critical for efforts to improve the diagnosis and treatment of CKD and other bioanalytical uses of creatinine. The benefits of enzymatic creatinine assays (ECr) over standard kinetic Jaffe assays (JCr) have been argued for over 10 years [5-6]. Despite these recommendations JCr assays are still commonly used. In a recent study at a German hospital center, 15% of patients diagnosed with acute kidney injury were detected with JCr assays alone [7]. Use of JCr assays alone are estimated to result in a 4% miscalculation rate of serum creatinine levels overall [8]. This error rate may be acceptable in a clinical lab screening setting due to the increased cost of ECr assays and the intrinsic biological variability of creatinine concentrations. However, studies have shown that minimal shifts in creatinine results can cause major alterations in GFR calculations and patient classification [6-9]. In drug development, precise determination of kidney function with biomarkers with the lowest analytical and biological variability is necessary to determine therapeutic efficacy and obtain approval by regulatory bodies.
Uses of Creatinine in Drug Development
Serum creatinine measurement is the gold standard endogenous biomarker for determination of GFR. Creatinine is a breakdown product of creatine phosphate from muscles produced at a fairly constant rate and removed by the kidneys [10-12]. The vast majority is removed by glomerular filtration with nearly no tubular reabsorption. Serum creatinine levels rise as glomerular filtration decreases and GFR estimating equations are used to correlate the serum concentration to GFR and determine the stage of CKD. Since many therapeutic compounds are eliminated through the kidneys, renal function can control drug clearance, efficacy, and safety. Therefore, regulatory agencies such as the Food and Drug Administration (FDA) and European Medicines Agency (EMA) have issued detailed guidelines for the requirements to determine GFR in new drug applications [13].
While many of these studies use clinical lab assays to determine creatinine, the uses of creatinine requiring bioanalytical validation and compliance are increasing. Clinical are only designed to distinguish healthy from abnormal values and thus therapeutics that require regulatory approval demonstrating improved renal function should follow bioanalytical guidelines. Measurement of urine creatinine is also a common assay used to normalize biomarkers of exposure, toxicity, and efficacy in urine [14]. This requires an accurate and validated creatinine assay rather than clinical lab measurement of creatinine. New biomarkers for metabolism, oncology, muscle mass also require bioanalytical measurement of creatinine in matrices such as urine, saliva, and serum [15-16].
Disadvantages of Conventional Assays for Creatinine
The Jaffe reaction (JCr) to measure creatinine originated in 1886 as one of the first clinical laboratory tests. Though it has evolved over the years, the basic reaction involves the reaction of creatinine with NaOH and picric acid in an alkaline solution to produce a colorimetric compound. The assay became automated in the 1960s into a kinetic assay where development of the chromogen is tracked over time [5]. The simple and cost-effective JCr assay has persisted to this day despite its significant shortcomings.
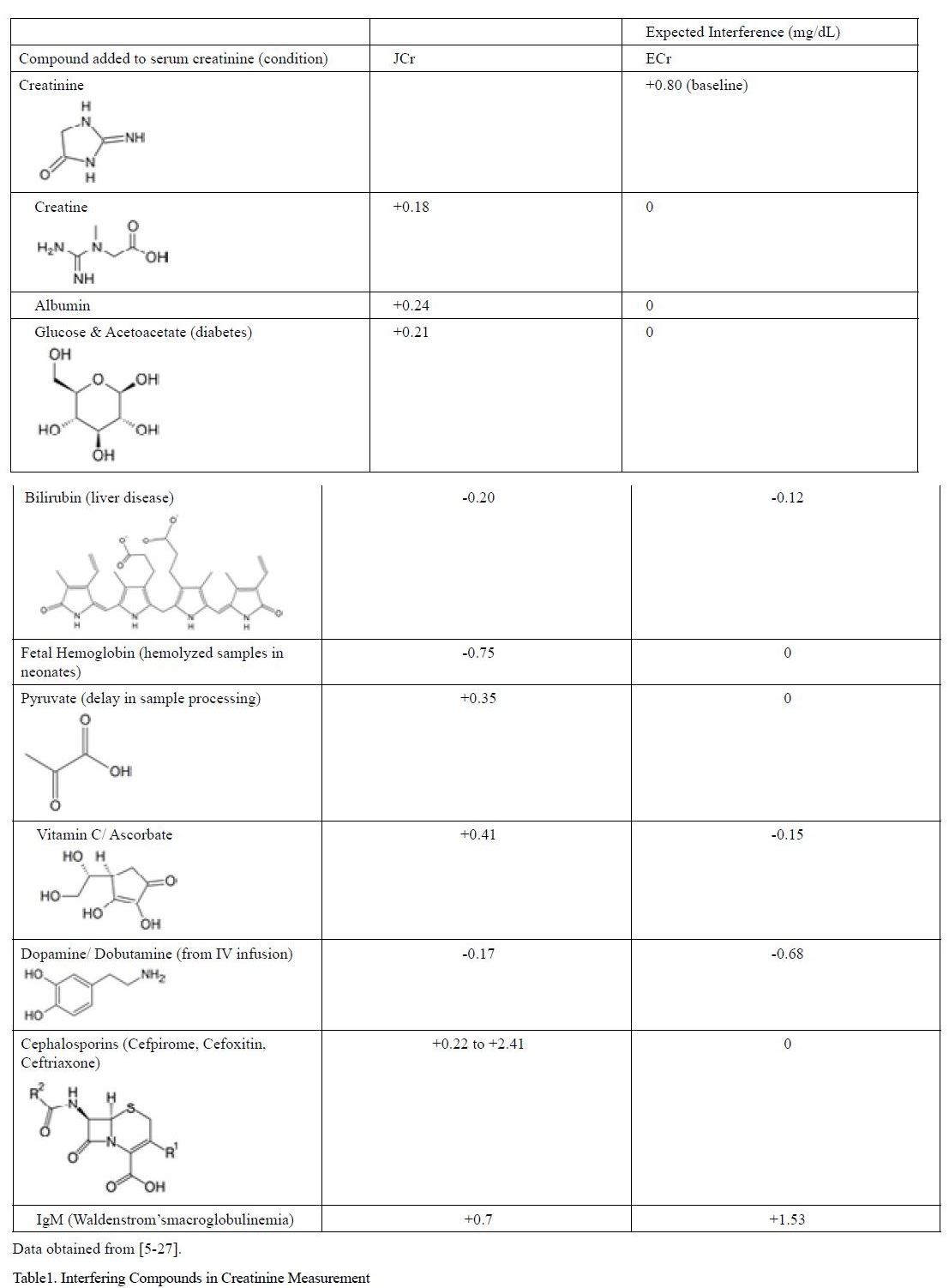
The primary concern with JCr assays is poor analytical specificity. Many substances commonly found in the serum of patients with many types of pathological conditions falsely elevate or decrease serum creatinine values leading to significant false positives and false negatives of kidney dysfunction. These compounds containing ketone, carboxyl, or amide groups similar to creatinine react with alkaline picrate to generate similar chromo gens. These compounds, summarized in Table 1 produce large changes in readings of creatinine and occur in large populations of patients such as those with liver disease, neonates, and those taking antibiotics. The interferences are especially important because many of the conditions that interfere with the assay, such as diabetes with elevated glucose and acetoacetates, are particularly prone to developing kidney disease [17-18].
Table 1:Interfering Compounds in Creatinine Measurement
A primary contributor for interference in JCr assays is cephalosporin antibiotics such as cefoxitin and cephalothin. The reaction product of alkaline picrate with creatinine and cephalosporin antibiotics has an overlapping molar absorptivity producing interferences as large as 213 µmol/L of creatinine in the JCr assay depending on the form of antibiotic used [5-19]. This is especially troublesome in developing countries where it is estimated that a staggering 44-97% of hospitalized patients are given antibiotics [20] and many individuals inappropriately self-medicate with antibiotics [20-21]. While newer forms of cephalosporin antibiotics have lower interference with JCr assays due to changing the equilibrium constants of the reaction with alkaline picrate, enzymatic assays see no interference with antibiotics [19].
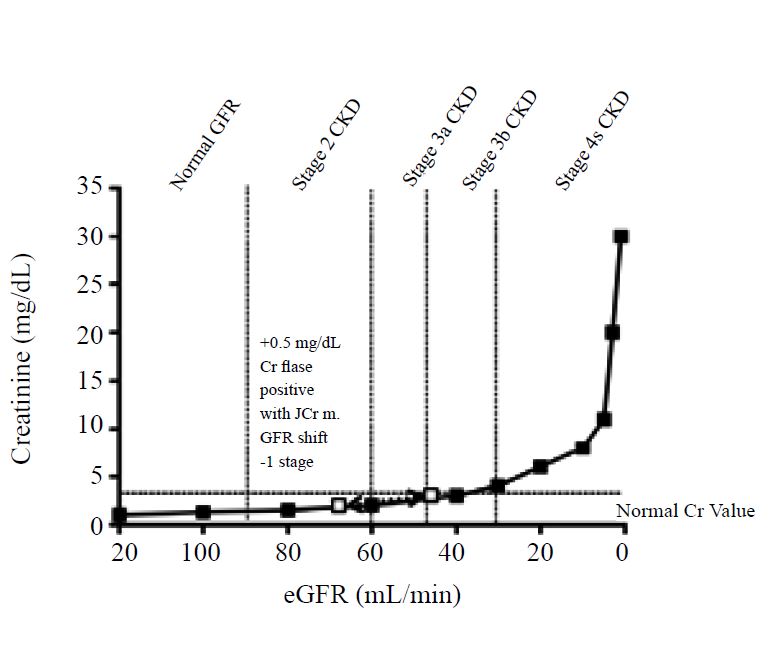
It is important to note that even minimal shifts in creatinine results can cause major alterations in eGFR calculations and the number of subjects classified as having different grades of reduced kidney function. As shown in Figure1, creatinine values do not increase past normal range until stage 3 CKD [22]. Therefore, small 0.05 mg/dL shifts in creatinine due to an inaccurate assay can cause an up to 2-stage shift in CKD leading to large numbers of false negatives and positives. For example, Klee et al. showed that a positive shift of 0.23 mg/dL (20µmol/L) creatinine tripled the number of individuals with an eGFR value less than 60 mL/min in a typical outpatient population [9].
Figure 1:Small shifts in Serum Cr cause large shifts in eGFRand lead to false positives and false negatives
Disadvantages of Modified and Compensated Jcr Methods
To improve the analytical performance of JCr assays, many agencies and vendors have modified the assay and launched efforts to improve and standardize these assays. First, international agencies have led efforts to standardize the reference material used in these assays to higher order creatinine reference material to improve precision of the assay across laboratories around the world. Most vendors have now calibrated their JCr assays to isotype dilution mass spectrometry (IDMS) reference methods [23]. However, IDMS and reference material traceability does not improve specificity of the assay and only ensures consistent precision between laboratories. Vendors have also introduced ?compensated? methods to address some of these specificity concerns that adjust calibrator concentrations. For example, a negative offset value can be introduced to compensate for positive interference caused by serum proteins. However this methodology is flawed because it determines an offset from an average interference from correlation studies and assumes that protein interference is constant across samples and linear across the calibration range. It does not account for changes in serum protein and creatinine ratios that occur in children, pregnancy, and the elderly. Methods have also been introduced to correct for bilirubin interference in JCr assays by reacting the bilirubin prior to analysis or ?rate-blanking? where a correction factor is determined by reacting the serum with NaOH alone after sample analysis [24-25]. However, this linear correction method does not account for the non-linearity of oxidation of bilirubin and thus does not fully correct for interference.
Advantages of Enzymatic Creatinine Assays
The enzymatic assay (ECr) originated in Australia to address growing concerns of false values from the JCr assays. Creatinine in serum is incubated with creatininase and creatinase to form hydrogen peroxide which can be measured spectrophotometrically as shown in the reaction below [26].
creatinine+H2O? (??creatininase ) creatine
creatine+H2O??(??creatinase ) sarcosine+urea
sarcosine+O2+H2O ?(??(sarcosine oxidase) ) formaldehyde+glycine+H2 O2
H2O2+4 aminophenazone+HTIB?(??POD ) quinone imine chromogen
This enzymatic cascade adds significant specificity to the formation of the chromogen and removes interference of the majority of compounds and physiological conditions that interfere with JCr assays. Although the enzymatic methods have been reported to have generally fewer interferences than the Jaffe methods, there have been reports of various substances that do interfere such as serum IgM and intravenously infused dopamine/ dobutamine [5,27-29]. Nevertheless, the growing body of evidence that ECr assays eliminate interference from many disease groups such as liver disease, diabetes, and bacterial infections supports their suitability for replacement of JCr assays in clinical and drug development settings.
In addition, and largely due to this improved specificity, the chemistry of the ECr assay allows improved accuracy. In a 2007 study, several laboratories determined that ECr had a median 0.1% deviation from nominal creatinine values while JCr assays showed a 16% median deviation from the nominal concentration [30]. This indicates that in situations where accuracy is crucial, such as when making dialysis or transplant decisions, ECr assays must be used when determining GFR. In addition, the deviation was found to be greatest at low concentrations of creatinine where early CKD is indicated (60 mL/min GFR) lending support to the belief that ECr assays should be used especially near the when GFR is expected to be near the 60 mL/min GFR early CKD decision limit or in drug development when detection of the early signs of CKD is crucial.
Finally, with the bioanalysis of any biomarker, increased emphasis is now placed on monitoring and controlling sample handling and collection as biological processes can have major effects on endogenous compounds [31]. To this effect, in addition to increased specificity and precision with ECr assays, the enzymatic reaction significantly improves variability from sample handling and collection [6]. In one study, delays in sample centrifugation built up chromogenic metabolites such as pyruvate and caused false increases in measured creatinine by three JCr assays of on average 50% and significantly changed CKD staging which did not occur affect ECr assays [32].
Disadvantages of ECr Assays
Despite the benefits of ECr, some disadvantages exist for ECr and the measurement of creatinine overall. In spite of the increase in vendors offering ECr assays, the cost is relatively higher than traditional methods ($0.50 per test for ECr vs. $0.10 per sample for JCr).
In addition, the benefits of using the ECr assay is limited primarily at concentrations of creatinine near the 60 mL/min GFR cutoff for kidney impairment where JCr is most inaccurate [8]. In addition, the difference in miscalculation due to use of JCr assays is often less than the biological variability, discussed below, which reduces the ultimate clinical diagnostic miscalculation resulting from using JCr assays [8]. Nonetheless, the accuracy benefits are expected to outweigh the increase in cost, especially in drug development and critical clinical diagnostic situations, because the increase in accuracy is near the critical decision threshold for the first stages of CKD.
Creatinine Biological Variability
The biological variability of creatinine is a result of the dynamics of creatinine production and elimination from the body. Specifically, muscle mass, which varies by age, ethnicity, and gender, significantly alters baseline levels of creatinine. While GFR estimating equations such as the Modification of Diet in Renal Disease (MDRD) equation account for these subgroups, the magnitude of change in muscle mass with these groups varies among populations and does not account for other physiological changes such diet, illness, inflammation, and deconditioning that affect muscle mass. To this effect, regulatory bodies and kidney disease agencies have started evaluating other renal biomarkers such as cystatin C, a small protease produced in all nucleated cells, as an eventual replacement for creatinine measurement [4]. Many comparison studies have shown cystatin C to outperform creatinine as an estimation of GFR and it is beginning to be used in clinical studies [33-34].
Recommendations for use of Creatinine in Clinical and Drug Development Settings
Even with newer biomarkers being validated for determination of GFR, creatinine will continue to be used for the foreseeable future. However, JCrassays continue to be widely used lending uncertainty to patient care, patient safety, and drug development decisions. Continued education of clinical laboratories and drug development clinical study coordinators is necessary to demonstrate the availability and benefits of the more specific biomarkers for renal function. It is also possible that more nuanced recommendations for specific use cases of each assay will aid in balancing cost and accuracy.
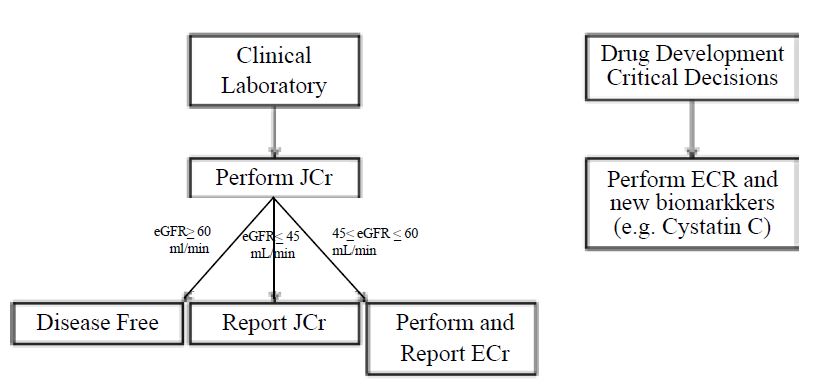
Based on the studies demonstrating that the JCrassay is most inaccurate around the 60 mL/min eGFR decision limit, we propose the following process for determining which assay should be used as shown in Figure2 [8]. For screening of kidney function in an clinical setting the JCr assay can be used initially. If results fall within the 45-60 mL/min, they should be repeated with ECr. Results outside of this zone can be used and reported with the original JCr result. However, for critical decisions where high accuracy is required (e.g. regulatory claim for improved kidney function, normalization or urine biomarkers) the use of ECr is highly recommended.
Figure 2:Assay recommendations for creatinine determination
A number of alternative analytical methods have been developed for drug development use including GC or LC/MS for measurement of creatinine several matrices including in serum, urine, saliva, and dried blood spots [35][16][36]. However the ECr assay remains a high throughput and accurate assay applicable to bioanalytical data. Comparison of a BMV validated LC-MS/MS method for serum creatinine with an ECr assay showed reported concentrations were near 1% of the LC-MS method while the JCr assay showed a bias of 12% [37]. In addition, ECr assays can be adapted to fully meet bioanalytical method validation criteria as shown below.
Bioanalytical Considerations to Support Drug Development Studies
Most assays for the measurement of creatinine are run in a clinical laboratory or in a laboratory using the principle of clinical laboratory testing. The primary purpose of a diagnostic assay is to distinguish diseased patients from healthy patients. We find that the assay design implemented to serve diagnostic purposes is not suitable for the measurement of eGFR in drug development, where priority is placed on accuracy across a large quantitation range, precision, and specificity with robust and consistent data across time and laboratories to determine the safety, mechanism of action, and pharmacokinetics.
Therefore, for drug development studies, most ECr assays acquired as research use only or in vitro diagnostic use commercial kits must be adapted to meet FDA bioanalytical validation guidelines and recent guidelines for biomarker validation [38-39]. The major differences in validation approaches used for drug development purposes following the FDA Bioanalytical Guidances vs. clinical lab validation approach is outlined in Table 2 which should be followed for adapting creatinine commercial kits.
Purpose |
IVD Biomarker Assay |
Drug Development Biomarker Assay |
Calibrators/ Standards |
Patient care - distinguish healthy from diseased |
Safety, mechanism of action, drug dosing |
Accuracy & Precision |
Often <6 calibrators |
>6 calibrators levels |
Quality Controls (QCs) |
One run per day over 5 days |
3-6 runs over multiple days needed |
Sensitivity |
QCs often in surrogate matrix only |
3-5 QC levels recommended including QCs in study matrix |
Stability Testing |
Only Limit of Detection typically defined |
Lower Limit of Quantitation must be evaluated for study samples |
Regulatory and Compliance Requirements |
Reagent Stability |
Analyte stability needed |
Purpose |
Methods validated following CLIA and CLSI guidelines |
FDA BMV 2013 draft guidelines for biomarkers, Critical Path Institute and industry white papers |
Table 2. Adaptations for validation of a biomarker assay from a commercial kit
For adapting a creatinine assay for use in drug development and subsequent regulatory submission data, the assay must be devised to have at least 6 non-zero calibration points. The calibration curve range should take into account the target range, including the expected LLOQ and ULOQ. The sensitivity of the assay must be carefully defined. Most clinical laboratory assays define their sensitivity at the limit of detection (LOD). For drug development, it is critical that the lower limit of quantitation (LLOQ) be defined for any assay. The quality controls (QCs) should also include the ULOQ and LLOQ in addition to the QCs that span the rest of the calibration curve and include QCs of endogenous creatinine in study matrix. Accuracy and precision should be performed with the intended biological matrix.
Of particular concern when using a biomarker for drug development is the presence of endogenous levels of the analyte. This should be taken into account during the method feasibility and development. Several approaches may be undertaken, including the use of surrogate matrix for the calibration curve. This approach is appropriate as long as the quantitation of the analyte of interest in the intended matrix is demonstrated by performing matrix effect and minimum required dilution analysis.
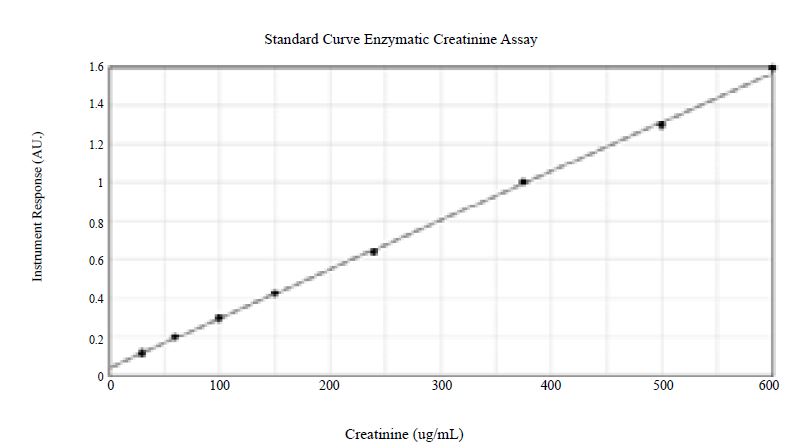
To illustrate the concepts in Table 2, we validated an ECr assay using a commercially available kit from Somru BioScience Inc. The kit was developed using the principle of FDA bioanalytical method validation (BMV) guidelines. The method uses eight non-zero calibration curve points as shown in Figure 3. The calibration curve was run using a surrogate matrix, and the QCs were in the intended matrix (urine). The BMV compliant validation demonstrates the appropriateness of the use of the surrogate matrix, showing an acceptable recovery of creatinine during accuracy and precision. Five levels of QCs across the entire range of the assay were utilized for determining accuracy and precision. Accuracy was shown to range from -4.3% to 0% bias, and precision was shown to range from 3.9% to 5.6%. The lower limit of quantitation was reliably shown to be 30µg/mL. Both long-term and short term stability bracketing the time period between samples collection and sample analysis was demonstrated. In addition, incurred sample re-analysis (ISR), as required by FDA BMV guidelines was performed on various studies and results were reproducible for greater than 90% of samples.
The data indicates that the validation of a commercial kit for bioanalytical purposes can be achieved. However due diligence must be done and the kit should be chosen carefully. The development of the assay and its historical performance should be carefully considered so that a successful context of use validation can be performed. In addition, use of an ECr method improves assay specificity, accuracy, and analyte stability to meet BMV criteria.
Figure 3:Enzymatic creatinine assay adapted with 8 calibrator concentrations for drug development validation
Conclusion
The enzymatic assay for creatinine achieves greater specificity, accuracy, precision, and stability compared to older JCr assays. Since creatinine values do not significantly rise until nearly half of kidney function is absent, small changes in reported creatinine concentrations causes large shifts in GFR calculations and risks missing detection of early kidney disease. The enzymatic assay attains the level of accuracy needed for regulatory and drug development decisions. For clinical lab settings, a two-step protocol using ECr assays when GFR is near the 60 mL/min achieves highest accuracy while managing costs.
Acknowledgements
The authors would like to acknowledge Richard Sukovaty and Jordan Donohoue at Celerion for assay validation and Muditha Balasuriya at Somru Bioscience for development of the enzymatic creatinine kit.
Executive Summary
Creatinine in drug development. Creatinine assays to measure GFR is typically measured using the picric acid based Jaffe reaction which is subject to interference from many substances commonly found in blood. These assays are not suitable for bioanalytical data.
Small shifts in creatinine cause large changes in calculated GFR which is especially problematic in situations where accurate reporting and tracking of GFR is necessary for therapeutic claims. The enzymatic assay for creatinine does not show interference and leads to improved accuracy. Adaptations of Creatinine Kits for Bioanalytical Data Research Use Only and In Vitro Diagnostic commercial kits that measure creatinine using the enzymatic reaction can be adapted to conform to FDA bioanalytical guidelines.
References
- (2014) Guidance for Industry and FDA Staff Qualification Process for Drug Development Tools Guidance for Industry and FDA Staff Qualification Process for Drug Development Tools. Food Drug Adm Cent Drug Eval Res.
- Group W, Fda US, Burkey J, Robert D, Martha D, et al. Points to Consider Document?: Scientific and Regulatory Considerations for the Analytical Validation of Assays Used in the Qualification of Biomarkers in Biological Matrices.1-54.
- Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W (2013) Urinary and serum biomarkers for the diagnosis of acute kidney injury: An in-depth review of the literature. Nephrol Dial Transplant. 28: 254-273.
- Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, et al.(2010) Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol 28: 455-462.
- Peake M, Whiting M (2006) Measurement of Serum Creatinine ? Current Status and Future Goals. Clin. Biochem Rev 27: 173-184.
- Panteghini M (2008) Enzymatic assays for creatinine: time for action. Clin. Chem Lab Med 46: 567-572.
- Haase M, Kribben A, Zidek W, Jurgen Floege, Christian Albert et al. (2017) Electronic Alerts for Acute Kidney Injury: A Systematic Review. Dtsch Arztebl Int 114: 1-8.
- Schmidt RL, Straseski JA, Raphael KL, Adams AH, Lehman CM (2015) A risk assessment of the Jaffe vs enzymatic method for creatinine measurement in an outpatient population. PLoS One 10: 1-21.
- Klee GG, Schryver PG, Saenger AK, Larson TS (2007) Effects of analytic variations in creatinine measurements on the classification of renal disease using estimated glomerular filtration rate (eGFR). Clin Chem Lab Med 45: 737-741.
- Levey AS, Eckfeldt JH (2017) Estimating glomerular filtration rate using serum creatinine. Clin Chem 63: 1161-1162.
- Levey AS, Inker LA, Coresh J (2014) GFR estimation: From physiology to public health. Am. J. Kidney Dis. 63: 820-834.
- Lopez-Giacoman S, Madero M (2015) Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol 4: 57-73.
- Paglialunga S, Offman E, Ichhpurani N, Marbury TC, Morimoto BH (2017) Update and trends on pharmacokinetic studies in patients with impaired renal function: practical insight into application of the FDA and EMA guidelines. Expert Rev. Clin Pharmacol 10: 273-283.
- Cone EJ, Caplan YH, Moser F, Robert T, Shelby MK, et al. (2009) Normalization of Urinary Drug Concentrations with Specific Gravity and Creatinine. J Anal Toxicol 33: 1-7.
- Gamagedara S, Kaczmarek AT, Jiang Y, Cheng X, Rupasinghe M, et al. (2012) Validation study of urinary metabolites as potential biomarkers for prostate cancer detection. Bioanalysis 4: 1175-1183.
- Angeles A, Christopher LJ, Wang Z, Arnold ME, Shen JX, et al. (2006) A validated LC ? MS / MS method for the quantitative measurement of creatinine as an endogenous biomarker in human plasma. Bioanalysis 8:1997-2005.
- Foundation NK (2007) Monitoring of Kidney Function in Patients with Diabetes. 30(8).
- Cavanaugh KL (2007) Diabetes Management Issues for Patients With Chronic Kidney Disease. Clinical Diabetes 25: 90-97.
- Kroll MH, Roach NA, Poe B, Elin RJ (1987) Mechanism of interference with the Jaffe reaction for creatinine Clin Chem 33: 1129-1132.
- Abdulah R (2012) Antibiotic Abuse in Developing Countries. Pharm Regul Aff Open Access 1: 1-3.
- Ab Rahman N, Cheong LT, Sivasampu S (2016) Antibiotic prescribing in public and private practice: a cross-sectional study in primary care clinics in Malaysia. BMC Infect dis 16: 208.
- Anderson CF, Jaecks DM, Ballon HS, De Palma JR, Cutler RE (1970) Renal Handling of Creatinine in Nephrotic and Non-Nephrotic Patients. Clin Sci 38: 555-562.
- Cheuiche AV, Soares AA, Camargo EG, Weinert LS, Camargo JL, et al. Comparison between IDMS-traceable Jaffe and enzymatic creatinine assays for estimation of glomerular filtration rate by the CKD-EPI equation in healthy and diabetic subjects. Clin Biochem 46: 1423-1429.
- Boot S, LaRoche N, Legg EF (1994) Elimination of bilirubin interference in creatinine assays by routine techniques: comparisons with a high performance liquid chromatography method. Ann Clin Biochem 262-266.
- O?Leary N, Pembroke A, Duggan PF (1992) A simplified procedure for eliminating the negative interference of bilirubin in the Jaffe reaction for creatinine. Clin Chem 38: 1749-1751.
- Crocker H, Shephard MD, White GH (1988) Evaluation of an enzymatic method for determining creatinine in plasma. J Clin Pathol 41: 576-581.
- McGill MR, Vijayan A, Trulock EP, Witt CA, Kohler GD, et al. (2016) Falsely Elevated Plasma Creatinine Due to an Immunoglobulin M Paraprotein. Am J Kidney Dis 68: 789-792.
- Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, et al. (2006) Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 52: 5-18.
- King LE (2016) Parallelism experiments in biomarker ligand-binding assays to assess immunological similarity. 8: 2387-2391.
- Blijenberg BG, Brouwer RJ, Baadenhuijsen H, Boerma GJM (1995) Creatinine and Surveys: An Assessment. Clin Chem Lab Med 33: 855-858.
- Wang J, Nowatzke W, Ma M. (2015) Current industrial practices and regulatory requirements to assess analyte and reagent stability using ligand-binding assays. Bioanalysis 7: 1371-1384.
- Shepherd J, Warner MH, Kilpatrick ES (2007) Stability of creatinine with delayed separation of whole blood and implications for eGFR. Ann Clin Biochem 44: 384-387.
- Roos JF, Doust J, Tett SE, Kirkpatrick CMJ (2007) Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children-A meta-analysis. Clin Biochem 40: 383-391.
- Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, et al. (2005) Cystatin C as a marker of GFR - History, indications, and future research. Clin Biochem 38: 1-8.
- Liu XY, Luo Y, Zhou CY, Peng A, Liu JY (2017) A sensitive and accurate method to simultaneously measure uric acid and creatinine in human saliva by using LC-MS/MS. Bioanalysis 9: 1751-1760.
- Randviir EP, Banks CE (2013) Analytical methods for quantifying creatinine within biological media. Sensors Actuators B Chem 183: 239-252.
- Ou M, Song Y, Li S, Gangyi Liu, Jingying Jia, et al. (2015) LC-MS/MS method for serum creatinine: Comparison with enzymatic method and Jaffe method. PLoS One 10: e0133912.
- Lee JW, Devanarayan V, Barrett YC, Weiner R, Allinson J, et al. (2006) Fit-for-purpose method development and validation for successful biomarker measurement. Pharm Res 23: 312-328.
- Arnold ME, Booth B, King L, Ray C (2016) Workshop Report: Crystal City VI-Bioanalytical Method Validation for Biomarkers. AAPS J 18: 1366-1372.